Case 1
- What are the urological indications for use of this medication?
- What is the mechanism of action when used in the bladder?
- What is the average duration of action?
- What are the contraindications to the use of this medication?
- What are the recommending dosing regimens?
Case 2
Reproduced with permission of Medtronic, Inc.
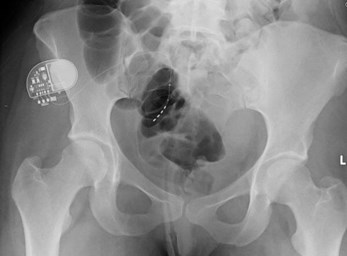
Image provided courtesy of Prof Hashim Hashim, Bristol Urological Institute.
- What is the device seen in the picture and the x-ray below?
- What are the indications for its use?
- What are the complications associated with the device?
- What is the evaluation process for the device?
- What is the success rate in patients with Fowler’s Syndrome?
Case 3
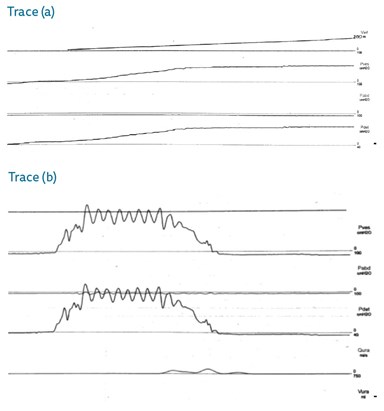
- Both of the above urodynamic traces were taken from patients with neuropathic bladders. What is the finding in each?
- What is the definition of bladder compliance and how is it calculated?
- What are the potential risks to the patient of the findings in these tracings?
Neurourology: answers
Case 1
- Idiopathic overactive bladder, neurogenic detrusor overactivity, bladder pain syndrome.
- The exact mechanism of Botulinum neurotoxin type A (BoNT/A) action is complex and not exactly known. The classical description involves binding of BoNT/A to presynaptic nerve terminals which cleaves SNAP-25 receptor protein from the soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complex. This results in the blockade of vesicular acetylcholine release and therefore stops neurotransmission at the neuromuscular junction. There is also increasing evidence that BoNT/A effects sensory nerve fibres and afferent signalling mechanisms, such as via purinergic pathways.
- The effect of intradetrusor botulinum toxin is approximately 9-12 months, therefore patients should be counselled regarding the need for repeat injections.
- Myasthenia gravis, Eaton-Lambert syndrome, breastfeeding, pregnancy.
- National Institute for Health & Care Excellence (NICE) guidelines recommend using 100 units as the initial dose in patients with idiopathic overactive bladder, increasing to 200 units if inadequate symptom relief. In patients with neurogenic detrusor overactivity the initial dose is 200 units; increasing to 300 units if inadequate symptom relief.
Case 2
- The Medtronic sacral nerve stimulator, sacral neuromodulation device, implantable pulse generator (IPG). Sacral nerve stimulation is also known as InterStim® Therapy.
- Overactive bladder, non-obstructive urinary retention and faecal incontinence
- Infection, pain at the implant site, leg pain, bowel disturbance, device explantation, lead migration, seroma, and haematoma. Battery depletion requiring replacement (usually between three to seven years depending on device settings), lack of efficacy and secondary loss of efficacy are other problems encountered.
- The first stage of SNS is an evaluation phase or test phase. The patient undergoes insertion of a temporary electrode with a unipolar electrode under local or general anaesthetic alongside a sacral nerve (generally S3) and connected to an extension lead to an external neurostimulator. Alternatively, a permanent tined lead can be inserted in the same position and tunnelled to the potential battery site, then connected via an extension lead to the external stimulator. The patient is then evaluated for two to four weeks with adjustments to the level of stimulation if required using a bladder diary. If the treatment is successful, the patient proceeds to implantation of a permanent IPG. If unsuccessful the wire is simply removed.
- Around 70% success [1].
Case 3
- Trace (a) shows poor bladder compliance at low volumes and (b) shows detrusor sphincter dyssynergia (discoordination between detrusor and sphincter function during voiding).
- Bladder compliance is defined by the International Continence Society as “the relationship between change in bladder volume and change in detrusor pressure”. It is calculated by dividing the change in volume by the change in detrusor pressure during that change in bladder volume. (ΔV/ΔP in ml/cmH2O)
- Poor compliance and detrusor sphincter dyssynergia places the patient at risk of upper tract dysfunction, bladder stone formation and an increased risk of urinary tract infections due to an increase in intravesical pressure and incomplete bladder emptying.
Reference
1. Swinn MJ, Kitchen ND, Goodwin RJ, Fowler CJ. Sacral neuromodulation for women with Fowler’s syndrome. Eur Urol 2000;38(4):439-43.





