Acute urinary retention is a common condition encountered in the emergency situation and is initially managed by urethral catheterisation. This is often performed by nursing staff or junior doctors. Post-obstructive diuresis (POD) is a specific entity which may occur post catheterisation and is a rare but potentially life-threatening condition if not recognised and managed appropriately.
The urology team, often consisting of an on-call surgical senior house officer or non-resident specialty registrar, must be adept at recognising patients at risk of POD, be aware of the parameters for its diagnosis and be able to manage it appropriately. Currently no clear guidelines exist on this. The aim of this article is to discuss the physiological mechanisms that cause POD, as well as setting out a clear algorithm for its diagnosis and management, which can easily be accessed by members of the multi-disciplinary team. This article is aimed at foundation doctors, core surgical trainees and junior urology registrars.
POD and transient haematuria are common complications following rapid decompression of urinary retention [1]. In 1951 Wilson et al. first presented clinical evidence of POD [2]. Studies have since demonstrated that a mild diuresis is usually a transient and appropriate physiological response to salt and water retention during the period of obstruction [3]. Within the literature, clinically significant POD has been defined as urine output of >3-4l/day or > 200ml/hour over two consecutive hours following catheterisation and reportedly occurs in 0.5 to 78% of patients with urinary retention [4,5].
It was initially thought that allowing gradual decompression of the bladder by limiting the initial drainage of urine and subsequently removing small quantities of urine at regular intervals would cause gradual reductions in intravesical pressure, and reduce the rate of complications [1,5-7]. Several studies have shown that this is not the case. Drainage of the first 100mls of urine decreases intravesical pressure by approximately 50% [8]. Isotope renographic studies demonstrate that removing only 60mls of urine causes rapid drainage from the upper urinary tracts and the onset of diuresis [9]. A recent randomised controlled trial comparing complication rates between gradual and rapid decompression showed there was no statistically significant difference between the two methods and gradual decompression did not reduce complication rates [7].
Mechanism
Proposed mechanisms for POD include excretion of retained water and salt, osmotic diuresis secondary to high levels of urea, production of diuretic and natriuretic peptides, loss of corticomedullary concentration gradient secondary to reduced urinary flow through the obstructed kidney and impaired tubular function, impaired response of the collecting duct to antidiuretic hormone and imbalance of sodium-regulating hormones [1,10,11]. The cause is likely to be a combination of the above mechanisms (Figure 1). These mechanisms are explained in more detail in Box 1.
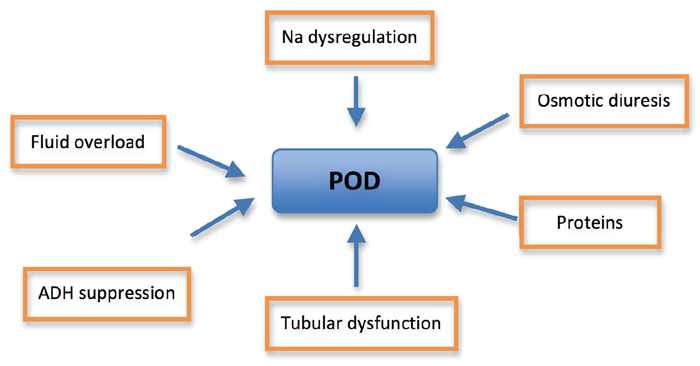
Figure 1: Mechanism of POD (Na = sodium, ADH = antidiuretic hormone).
Box 1: Physiology and definitions relating to the mechanism of POD.
Physiology and definitions:
Osmotic diuresis: Increased urinary excretion secondary to osmotically active solutes within the renal tubules. These substances exert an osmotic pressure within the tubule causing water retention in the lumen. Examples include increased urea in POD and increased glucose in diabetes mellitus.
Natriuretic peptides: Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) increase sodium excretion, and therefore water, by the kidneys. They act to decrease overall blood volume [14].
Glomerular function: Glomerulus consists of capillaries within Bowman’s capsule. Filtration and formation of urine occurs at the glomerulus [15].
Renal tubule function: Selective reabsorption or excretion of water, various ions and solutes to maintain normal volume and electrolyte composition of body fluid [15].
Salt losing nephropathy: Excessive loss of urinary sodium secondary to renal tubular dysfunction [16].
Tubular dysfunction and recovery is thought to have an instrumental role in the transient physiological response of POD. Urinary output and electrolyte excretion are maximal within 24 hours and usually stabilise by day 14. Glomerular filtration rate studies suggest the early improvement is probably due to tubular recovery. However, a late glomerular recovery phase has also been identified [12].
It was previously thought that clinical presentation does not predict which patients will have POD. There was no evidence correlating initial creatinine values, or other biochemical and clinical markers, with the severity and duration of diuresis [1]. However, a study showed that patients at most risk of significant POD were either fluid overloaded, had severe renal impairment or central nervous system (CNS) symptoms [13]. CNS symptoms to look for are those associated with hyponatraemia such as nausea, malaise, headache, irritability and confusion. A recent bi-centre retrospective observational study of ITU patients with severe post-renal acute kidney injury identified higher serum creatinine and higher serum bicarbonate as early independent predictors of POD.
Management
There is a distinct lack of evidence regarding the pathophysiology and optimal management of POD in the recent urological literature, which is reflected in the absence of validated guidelines nationally or internationally on the subject. Some departments have developed their own local management guidelines. Our aim is therefore to propose a concise and stepwise management algorithm on how to diagnose and manage POD based on current evidence (Figure 2).
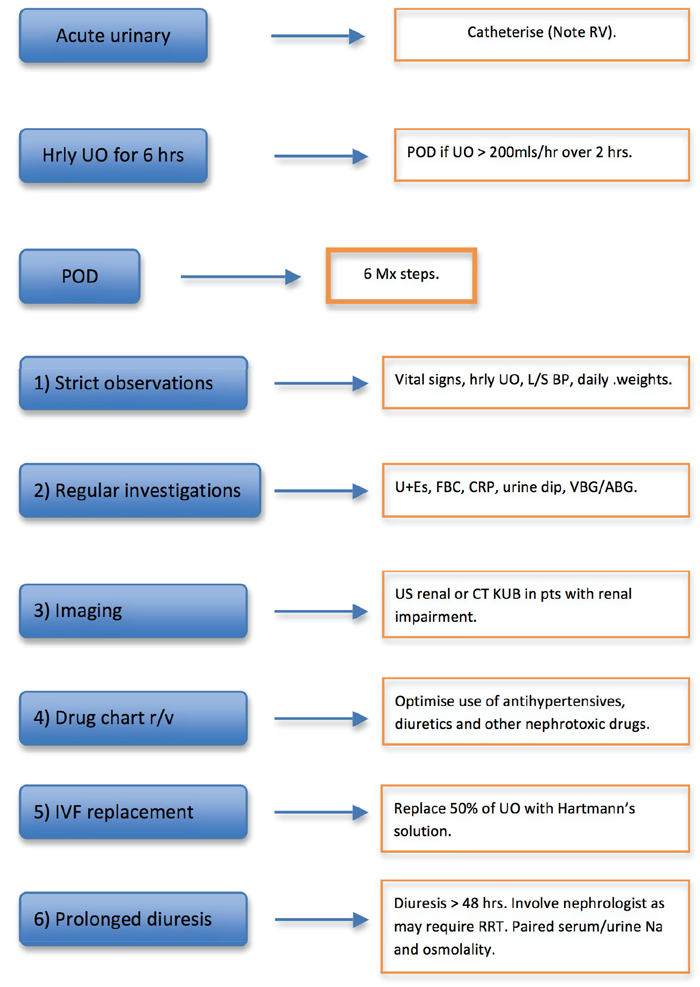
Figure 2: Management algorithm for POD. RV = residual volume. UO = urine output. L/S = lying / standing. U+Es = urea & electrolytes. FBC = full blood count. CRP = C-reactive peptide. VBG/ABG = venous / arterial blood gas. US = ultrasound. IVF = intravenous fluid. NaCl = sodium chloride. RRT = renal replacement therapy.
As with any presenting complaint, a thorough history and examination to elicit the cause of urinary retention is paramount. It is important to explore red flag symptoms and signs, and exclude sinister causes of urinary retention such as cauda equina syndrome. One must also be aware of associated management issues such as sepsis, haematuria and acute kidney injury.
Every patient with acute urinary retention needs to be catheterised. Following catheterisation the initial drained volume within 10-20 minutes of catheterisation needs to be accurately recorded. It is anecdotally recognised amongst urologists that a small volume of urine drained immediately after catheterisation is unlikely to precipitate POD. Most units use a cut off between 750-1000mls to monitor for POD, although the evidence base for this is not clear. Urinary dipstick with or without urine microscopy must be performed. Urine output should be monitored hourly for six hours. Usually, as a mild diuresis is a self-limiting physiological response that continues until an euvolaemic state is achieved [10], non-invasive bedside investigations and routine observations are normally all that is required. POD is diagnosed if urine output is greater than 200ml/h over two consecutive hours.
If POD is diagnosed, in addition to routine observations, a fluid balance (‘input / output’) chart for recording hourly fluid intake and urine output, lying and standing blood pressure to monitor postural hypotension and daily weight needs to be accurately recorded [6]. Regular investigations should include daily measurement of urea, creatinine and electrolytes to ascertain renal function and determine if the patient has acute kidney injury (AKI). Concomitant urine infection, if suspected, would be ruled out by checking the full blood count, CRP and urine dipstick analysis.
Patients are preferably managed by oral fluid administration [5]. Intravenous fluids are reserved for patients with a postural drop in blood pressure, haemodynamic instability or diuresis leading to electrolyte disturbances and the elderly who may not be able to reliably maintain the required fluid intake, particularly overnight [5,6]. Most recommend replacing half to two-thirds of the urine output over a similar time period [13,10]. Excessive fluid replacement may actually perpetuate and prolong the diuresis [6,11,13]. Whilst the literature suggests the optimal type of fluid for intravenous fluid replacement is 0.45% sodium chloride [10], in practice, Hartmann’s solution is often used.
POD patients with renal impairment should have immediate imaging of the renal tract in the form of ultrasound to observe the degree of hydronephrosis [17-19] and to delineate further imaging and investigations. Renal ultrasound will aid in the diagnosis of high pressure retention when biochemical monitoring of renal function is inconclusive. Patients with high pressure retention require either a long-term catheter or definitive surgical management. Ultrasound is an essential investigation in patients with a prolonged period of diuresis or kidney injury as exclusion of other post-renal causes of kidney injury is required. When worsening renal function is encountered in spite of conservative measures it is useful to obtain an opinion from the renal physicians. Blood gas analysis provides valuable information on acid and bicarbonate levels, not only for predicting severity of POD [3] but also for monitoring purposes. In cases of severe diuresis, correction of acid-base levels in liaison with renal physicians and intensivists may be necessary. Anti-hypertensives, diuretic therapy or other nephrotoxic medication may need to be temporarily suspended if patients have profound or worsening AKI, in particular acidosis and electrolyte imbalance, which may be confounded by nephrotoxic medications [6].
Prolonged or refractory diuresis should be considered when the patient continues to have diuresis for greater than 48 hours despite supportive treatment. At this point, nephrologists and intensivists should be involved, as patients may need renal replacement therapy. Paired serum / urine sodium and osmolality helps to determine if it is a concentrating defect or a salt losing nephropathy and guides with fluid replacement. Pathologic salt losing nephropathy requires normal (0.9%) saline and may even require hypertonic (3%) saline [10].
Once the acute POD has been managed, the patient can be assessed for definitive treatment of their urinary symptoms in an elective setting. Patients who have had a high pressure urinary retention / POD must not have their catheters removed unless a definitive surgical procedure for management of their bladder outflow obstruction is performed. Conclusion POD is a common complication of rapid decompression of urinary tract obstruction. Usually, it is a self-limiting and physiological response that requires supportive care.
References
1. Nyman MA, Schwenk NM, Silverstein MD. Management of Urinary Retention: Rapid Versus Gradual Decompression and Risk of Complications. Mayo ClinProc 1997;72:951-6.
2. Wilson B, Reisman DD, Moyer CA. Fluid balance in the urological patient: disturbances in the renal regulation of the excretion of water and sodium salts following decompression of the urinary bladder. J Urol 1951;66:805-15.
3. Jones DA, George NJ, O’Reilly PH, Barnard RJ. Reversible hypertention associated with unrecognized high pressure chronic retention of urine. Lancet 1987;1:1052-4.
4. Hamdi A, Hajage D, Van Glabeke E, et al. Severe post-renal acute kidney injury, post-obstructive diuresis and renal recovery. BJU International 2012;110(11c):E1027-E1034.
5. Ahmed M, Abubakar A, Lawal AT, et al. Rapid and complete decompression of chronic urinary retention: a safe and effective practice. Tropical Doctor 2013;43:13-16.
6. Foster MC, Upsdell SM, O’Reilly PH. Urological Myths. BMJ 1990;301:22-9.
7. Boettcher S, Brandt AS, Roth S, et al. Urinary Retention: Benefit of Gradual Bladder Decompression – Myth or Truth? A Randomised Controlled Trial. Urol Int 2013;91:140-4.
8. Christensen J, Ostri P, Frimodt-Moller C, Juul C. Intravesical pressure changes during bladder drainage in patients with acute urinary retention. Urol Int 1987;42:181-4.
9. George NJ, O’Reilly PH, Barnard RJ, Blacklock NJ. Practical management of patients with dilated upper tracts and chronic retention of urine. Br J Urol 1984;56:9-12.
10. Taneja SS. Complications Of Urologic Surgery: Prevention and Management. 4th Edition. Philadelphia, USA; Saunders Publishing; 2009.
11. Kalejaiye O, Speakman MJ. Management of Acute and Chronic Retention in Men. European Urology Supplements 2009;8:523-9.
12. Mundy AR, Fitzpatrick JM, Neal DE, Geroge NJR. The Scientific Basis of Urology. 2nd Edition. 2010; Taylor & Francis; 2004.
13. Vaughan ED, Gillenwater JY. Diagnosis, characterization and management of post-obstructive diuresis. J Urol 1973;109:286-92.
14. Widmaier EP, Raff H, Strang T. Vander’s Human Physiology. 11th Edition. UK; McGraw-Hill Higher Education; 2007.
15. Kumar P, Clark M. Clinical Medicine. 7th Edition. Philadelphia, USA; Saunders Elsevier; 2009.
16. Accessed from
http://www.merckmanuals.com/
professional/endocrine_and_metabolic
_disorders/electrolyte_disorders/hyponatremia.html
17. Noble VE, Brown DFM. Renal Ultrasound. Emerg Med Clin N M 2004;22:641-59.
18. Madersbacher S, Alivizatos G, Nordling J, Sanz CR, Emberton M, Rosette J. EAU 2004 Guidelines on Assessment, Therapy and Follow-up of Men with Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Obstruction (BPH Guielines). European Urology 2004;46:547-54.
19. Speakman MJ, Cheng X. Management of the Complications of BPH/BOO. Indian J Urol 2014;30(2):208-13.
TAKE HOME MESSAGE
-
POD is usually a transient and appropriate physiological response to salt and water retention during the period of obstruction.
-
Gradual decompression does not reduce complication rates. Rapid and complete decompression is safe and effective, less laborious and clinically practical. It should become standard practice.
-
Independent predictors of POD include higher serum creatinine and higher serum bicarbonate.
-
Management of POD requires supportive care with close monitoring and fluid replacement. Special attention should be given to elderly patients and those with multiple co-morbidities.
-
Use intravenous fluids in patients who cannot replace their fluid losses orally, those with a postural drop in blood pressure, haemodynamic instability or diuresis leading to electrolyte disturbances.
Declaration of competing interests: None declared.







