Percutaneous nephrolithotomy (PCNL) is now the gold standard approach to treating large renal stones. Since its development in the 1970s, it has undergone a series of refinements that could only have been possible with the symbiosis of both radiological and urological advancements.
Originally described by Fernstrom and Johansen on three patients in 1976 [1], early pioneers included Alken, Marberger and Wickham in Europe, with Clayman, Smith and Segura across the Atlantic in the USA.
Over the past 20 years, improvements in nephroscopes, guidewires, stents and stone fragmentation devices have led to the constant refinement of the technique with an ever-increasing range of stone sizes applicable for its use.
Despite these advances, PCNL still represents a high-risk operation associated with morbidity and even occasionally mortality. It is often the preference and clinical decision-making of the operating surgeon that will define the approach, position, access, technique and drainage postoperatively. With so much at stake and such variation in practice, it is important to carefully select, work up and approach cases with the method that feels most comfortable to the operating team.
Patient selection
In many clinical scenarios, the widespread use of flexible ureterorenoscopy with vastly improved optics, irrigation and the advent of the ‘disposable scope’ has made an inevitable encroachment into the PCNL’s territory for tackling larger renal stones. In tandem with this, the miniaturisation of the PCNL has continued to gain ground over the past 10 years; it is now considered a reasonable option for smaller-than-traditional PCNL stone sizes (such as in patients with refractive stent symptoms, who are keen to avoid a two-phased flexible ureterorenoscopy).
Furthermore, the range of approaches available to miniaturisation (from 4.8Fr-26Fr), varying opinions on nomenclature and early data make it difficult to ascertain the best approach / size, with proponents putting forward data on the superiority of their chosen technique / size. Miniaturised PCNL is often heralded as having similar benefits to a ‘totally tubeless’ PCNL which had its inception in the treatment of stones in the paediatric population – described in 1997 by Helal et al., using a 10Fr paediatric cystoscope [2]. There are a number of different nomenclatures used to describe sizes less than the more traditional 26Fr–28Fr, but thus far there has been no consensus. Therefore, in the interests of clarity, it is often easier to use the tract size rather than the particular title attributed to the procedure (‘micro’, ‘ultramini’, ‘supermini’, etc.).
Whilst there is a definite role for miniaturised PCNL, it currently is not in a position to replace the standard PCNL. Ruhayel et al. came to the broad conclusion that, whilst stone-free rates are comparable, blood loss is significantly reduced but procedure duration is increased [3]. Huusmann et al. also identified the increase in intra-renal pressures particularly below 14Fr as a risk factor for renal damage – especially when considering the increase in operative time [4].
The traditional variables such as a thin patient with low riding kidney (with no predisposing chronic kidney disease), multiple large renal stones in multiple calyces or large lower pole stones (>15mm) could also be considered to fall within the scope of miniaturised PCNL. Therefore, in the context of this article, we felt it was most appropriate to consider the standard approach to PCNL (26-28Fr) to prevent confusion. In this respect, the perfect case for a standard PCNL (i.e. one that was not suitable for a miniaturised procedure), a staghorn calculus.
The cases with particular challenges to consider
The most important consideration for achieving consistently successful outcomes in PCNL with minimal complications is through correct and safe patient selection. The decision to choose a PCNL for a stone-bearing kidney is based upon various factors including stone size and composition, patient comorbidities and the best approach for the percutaneous tract to access the stone(s). These decisions can be difficult and in many units, particularly those used to managing complex stones, the decision is best made through the utilisation of a stone multidisciplinary team (MDT) meeting involving urologists and uro-radiologists, with microbiologist input adding further value for the perioperative antibiotic prophylaxis and treatment.
However, there are some instances or circumstances where the risks of the operation may outweigh the potential benefits. Uncorrected coagulopathy is one such condition and is contraindicated in any percutaneous intervention including PCNL.
Similarly, uncorrected sepsis is a contraindication for PCNL, however in the complex stone harbouring multiple organisms held behind obstructed calyces, it may be impossible to completely clear the patient of bacteria without relieving the obstruction and / or treating the stones. In these instances, a percutaneous puncture may result in the discharge of pus. In such cases the safest approach is to deploy a nephrostomy and cover adequately with antibiotics. The subsequent management often depends on the patient’s response upon decompression. It is often sensible to proceed to the operation whilst antibiotic cover is on board and sensitivities have been cultured with close microbiological support. Other factors that the MDT may consider to be unfavourable are listed in Tables 1 and 2.
Table 1: Anatomical factors substantially increasing difficulty of the PCNL [5].
Horseshoe kidney
Results in more inferior, medial and malrotated kidneys that have a high ureteric insertion. Generally require upper pole access as lower pole is more anterior than in normally positioned kidney.
Mal-rotated kidney
The rotation of the kidney results in the change in position of the avascular plane between anterior and posterior segmental renal arteries (Brodel’s line).
Pelvic kidney
The ectopic position often leaves it in very close proximity to bowel and major vessels, which may have an anomalous supply. The kidney’s presence deep in the pelvis precludes access from the prone approach, requiring an ultrasound-guided, supine approach.
No retrograde access
Seen in patients with urinary diversions, or non-reconstructable urethras. Since there is no retrograde access, the ureteric catheter cannot be deployed to provide a retrograde pyelogram to assist in defining the pelvicalyceal anatomy for fluoroscopic guided puncture / wire insertion.
Spinal deformity
The grossly abnormal anatomy seen in severe scoliosis or in spina bifida patients makes for some of the most difficult punctures and operations. This can be partly due to the abnormal location of the kidneys and their close apposition to other organs. An ultrasound-guided approach, with a modified patient position (that is sympathetic to the patient’s natural ‘position’) allows the least risk to organ systems with shortest tract length.
Narrow-necked calyceal diverticulum
With challenging, or even impossible, access into the collecting system [6], this means that dilatation often will occur with no safety wire secured down the ureter or within the collecting system.
Table 2: Other less significant risk factors.
Branched collecting system
Particularly when stones are in multiple calyces and therefore torque manipulation of the scope is difficult.
Tight infundibulae
Subsequent dilatation causes rupture of the calyceal neck, leading to extravasation at an early stage of the operation.
Significant obesity
This makes tract and sheath manipulation more difficult (a longer sheath and scope may be required).
Previous PCNLs or recurrent renal infections
These predispose to scarred peri-nephric tissue – this can cause significant difficulty when attempting to gain access and tract dilatation.
Hypermobile kidney
Wire access may not be too difficult, however dilatation is often challenging (as the kidney moves away on manipulation so that the parenchyma cannot be dilated). Recommendations include dual puncture and the deployment of an anchoring wire to reduce movement and dilatation over one wire only.
Patient-related comorbidity
Patients with staghorn stones suitable for PCNL can often be quite medically unfit; this will therefore translate into a higher postoperative risk of complications. Dore et al. found that the conditions of diabetes and cardiovascular disease were significantly correlated with an increased complication rate [7]. Similarly, Clavien-Dindo grade III classification complications were seen to be 2.7 times more common in patients with diabetes and hypertension [8] undergoing PCNL. However, it is widely accepted that large stones have a greater rate of growth [9] and there is an associated risk of mortality with untreated staghorn calculi [10]. Therefore, the approach would be to mitigate the operative risk by multi-specialty involvement throughout the perioperative episode.
Table 3: Summary of suprine versus prone position.
Anaesthetic
Supine: No significant positional concerns.
Prone: Requires awareness of pressure areas (orbits), spinal fixation upon prone rotation, decreased venous return.
Irrigation
Supine: Lower calyceal pressure – reduced para-renal reflux and easier escape of fragments.
Prone: Elevated calyceal pressure – but arguably improved vision.
Operative time
Supine: Shorter time spent positioning patient.
Prone: Increased time to transfer (from lithotomy position to prone). But techniques to shorten transfer time include ureteric access in the ‘frogs legs’ or prone ‘split-legs’ position.
Radiation
Supine: Reduced radiation for fluoro-assisted puncture
Prone: Imaging column directly over access area so less exposure to the surgeon’s / radiologist’s hands.
Obesity
Supine: Longer tract length.
Prone: Shorter track length.
Access
Supine: Often lower pole access due to position of kidney.
Prone: Upper pole access easier with posterior more medial calyces – along brodel’s avascular plane – reducing risk of bleeding.
Complication rates
Supine: No difference.
Prone: No difference.
Stone-free rates
Supine: No difference.
Prone: No difference.
The surgery
Positioning
The question of supine vs. prone positioning is often debated, with the outcome largely agreed to be a stalemate [11,12] – see Table 3 for a summary of supine versus prone position. Kamphuis et al. from the Clinical Research Office of the Endourological Society (CROES) study group state worldwide there is still a preference for the prone approach (80% perform prone) [13]. Perhaps the most important factor when determining a successful approach is the experience of the operating team.
Access
It is imperative that puncture takes place through the centre of the calyceal papillae to avoid damage to the interlobar and arcuate branches of the renal artery. It has been reported by Sampaio et al. that injury to interlobar vessels occurs in around 67% of upper pole and 13% of lower pole punctures [14].
Although fluoroscopy allows identification of the desired calyx for puncture (through the use of retrograde pyelography via an indwelling ureteric catheter), it is limited by its inability to see adjacent organs, such as pleura and bowel [15]. Additionally, ultrasound-guided puncture provides real-time, bi-planar tracking of the route of the puncture into the calyx; posterior calyces can be easily identified and the shortest route (thereby the least disruption to parenchyma) can be achieved. The 2017 meta-analysis by Liu et al. points to a shorter access time, higher success rate at first puncture and a reduced complication rate [16]. However, with benefits from both modalities, the combined approach of both fluoroscopy and ultrasound allows for the most efficient form of access. Bayles et al. report that 70% of the tracts in their pan-UK questionnaire were performed by radiologists, with the rest obtained by urologists [17].
“Meticulous prior planning is invaluable in mitigating risk; however, despite the best planning and preparation both intra and postoperative complications can still result”
Tract formation
Tract dilatation can be made either with serial dilators (metal or plastic) or ‘one-step’ balloon dilatation; there is no conclusive evidence to a superior technique with advocates for both. Serial dilators allow a rigid scaffold for sequential dilatation and prevents ‘waisting’ sometimes seen in balloon dilatation. This technique it is of particular use when dealing with scarred perinephric tissues; conversely balloon dilatation takes a shorter period of time, in one step and with limited manipulation of tissues.
Intraoperative decision-making
The ideal tract runs along the axis of the infundibulum of the calyx and allows minimal manipulation with scope angulation. An upper pole puncture is preferred to allow the greatest opportunity to access the greatest number of calyces.
In patients with complex stone burden, multiple tracts may be required to clear. However, this also increases the bleeding risk from 7.6% to 18.5% [18]. Lu et al. suggested that colour Doppler could be used to assist in ultrasound-guided punctures to reduce the risk of damage to vasculature [19].
Intraoperative bleeding can obscure vision and absorb light. The operative vision can be improved by repositioning the access sheath either by advancing the sheath or identify the area of bleeding and applying tamponade.
Mariappan et al. showed that in obstructed systems the preoperative urine culture does not accurately predict risk of postoperative sepsis [20]. Therefore, the recommendation would be to send intraoperative pelvic urine and stone for culture to assist in the postoperative period (>24 hours).
Table 4: Key conditions that influence the decision for drainage.
-
Significant blood loss or collecting system injury.
-
Residual stone burden – and therefore the potential for a second phase flexible ureteroscopy (in which case a stent might help) or a second look nephroscopy (in which case a large calibre nephrostomy tube is helpful).
-
Length of operative time.
-
Size of access – a miniaturised PCNL lends itself to avoiding drainage tubing due to the relatively atraumatic puncture and dilatation.
-
Ease of procedure – ‘lift out’ of stone versus protracted fragmentation of a large / infected stone (the former suitable for no nephrostomy whereas the latter would certainly benefit from one)
Drainage (Table 4)
A variety of postoperative nephrostomy tube sizes are in use (6‑28Fr), with proponents for and against the multitude of drainage tubes to choose from; the type is largely specific to unit or surgeon preference. The ideal characteristics include an internal calibre appropriate for adequate drainage, strength, the ability to provide adequate tamponade on renal parenchyma, tolerated by the patient and easy enough to insert and remove, but also one that can provide access for a second-look procedure if so required.
The term ‘tubeless’ PCNL is often used when a ureteric stent is inserted at the end of the procedure but without any percutaneous drainage. However, Zhao et al. showed that patients with antegrade stents have a lower length of stay compared to those with nephrostomy drainage only (1.9 vs. 3.2 days) [21]. In the immediate postoperative period of seven days, patients with stents had significantly worse quality of life scores (on the Wisconsin Stone Quality of Life questionnaire), when compared to those given nephrostomies (which were subsequently removed by day seven) – presumably due to the high prevalence of bothersome stent symptoms.
Totally tubeless PCNL is associated with a reduced length of stay and improved postoperative analgesic requirements. A solitary stone, as a ‘lift-out’ case (thereby removing the need for a relook procedure), with one tract (reducing the risk of bleeding) in an uncomplicated kidney and patient, lends itself to a totally tubeless approach. This has been shown as a safe option in the carefully assessed patient by Xun et al., who noted that there were no significant differences in stone-free rate, postoperative fever or transfusion rate in patients without nephrostomy tubes compared with patients with one. [22].
Complications and postoperative management
Meticulous prior planning is invaluable in mitigating risk; however, despite the best planning and preparation both intra and postoperative complications can still result. Transient fever was noted in 3.5% of 315 patients by Osman et al. [15], with infection related complications (urinary tract infection / sepsis / fever) as the main reasons for readmission across multiple studies. Armitage et al. describe the readmission rate as 9%, with 30-day mortality on average at 0.2% [23].
The CROES PCNL study group suggested that higher volume centres had better outcomes both in terms of stone-free rate as well as associated complications [24]. However, this was not a view held by the Clinical Effectiveness Unit at the Royal College of Surgeons who looked specifically at the UK hospital episode statistics database and found significance only in a reduced length of stay at higher volume units [25].
Renal pelvic injury
Intraoperative damage to the collecting system manifests by the observation of renal sinus or peri-nephric fat, akin to fine ‘yellow cobwebs’. It is reported in up to 7% of procedures [26]. When this occurs, the operative procedure should be kept as brief as possible. Reducing the irrigating pressure during the operation and ensuring low drainage pressure postoperatively with the placement of a ureteric stent and urethral catheter or a nephrostomy is the recommended management – most perforations heal within 72 hours [27].
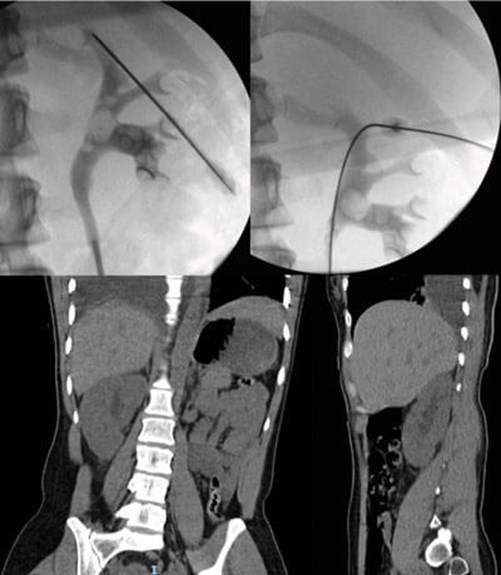
Figure 1: Right-sided hydrothorax post-supra 12th access for PCNL.
Pleural compromise
This is a rare complication but one that is important to identify early (Figure 1). The risk of injury increases for punctures that are supracostal especially those that are medial. Identification requires a degree of clinical suspicion; intraoperative signs include elevated pressures on ventilation and hypoxia. However, symptoms of respiratory compromise and pain do not usually declare themselves until the nephrostomy tube is removed (due to the tamponade effect of tube against pleura). Treatment is a rapid chest CT and urgent insertion of a chest drain.
Ultrasound-guided puncture allows assessment of the pleura prior to puncture and shows movement in real-time. Despite this, supracostal access has a 10-15% risk of intrathoracic complications, compared to 1.5-4.5% with subcostal access [27]. Whilst a coiled nephrostomy is often enough to drain fluid and allow the pleura to heal, in cases where there is spillage of debris or significant empyema a formal chest drain may be required.
Arterial injury
Transfusion rates vary widely between series and depend on several factors including: number of tracts, length of procedure and surgical experience. Aspirin, which was typically ceased preoperatively has now been shown to be safe to continue [28]. Other antiplatelet agents (such as Clopidogrel) and anticoagulants need to be stopped, with appropriate bridging plans instituted perioperatively.
Arterial injury is often directly related to access; posterior calyceal access along Brodel’s avascular plane (the watershed of the anterior and posterior segmental renal arteries) avoids blood vessels that course alongside the infundibulum – so that the tract runs parallel to and not across arteries and thus reducing the risk of arterial injury.
If stones cannot be easily reached it is important to avoid excessive torqueing of the rigid nephroscope and / or its sheath which can cause intraoperative venous injury, which will declare itself through a deterioration in vision due to bleeding. This may be avoided by the appropriate / judicious use of multiple tracts or the use of a flexible nephroscope, to access difficult calyces and avoid the need for a further puncture / dilatation.
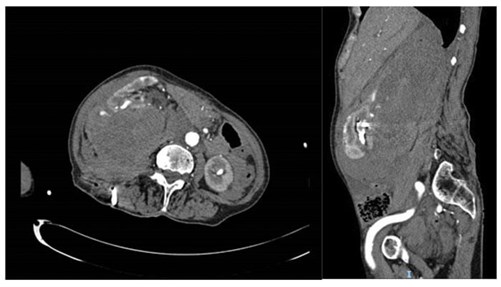
Figure 2: Post-PCNL bleed into retroperitoneum.
Postoperative bleeding (Figure 2) can be usually resolved by a short period of nephrostomy clamping (10-15 minutes), especially if arising from venous vasculature. However, it is prudent to have a high clinical incidence of suspicion in such cases and proceed to renal angiography with angioembolisation. The postoperative transfusion rate varies widely in the literature, with the British Association of Urological Surgeons quoting the national average as 2.1% [29].
Whilst initially it might be reassuring, the absence of any blood-stained urine in the urethral catheter may indicate ipsilateral ureteric obstruction (either from a stone fragment, ureteric oedema or a blood clot) as the clear urine seen in the bladder represents normal urine from the contralateral kidney. Within this scenario, persistent and worsening haematuria through the nephrostomy is an indicator of clot-colic, for which additional vigilance for bleeding is needed. In any of these situations, imaging (by CT KUB, nephrostogram or CT angiogram as appropriate) will identify the level and cause of obstruction and allow onward management as required, including the opportunity to deploy an antegrade stent via the nephrostomy tract (Figure 3).
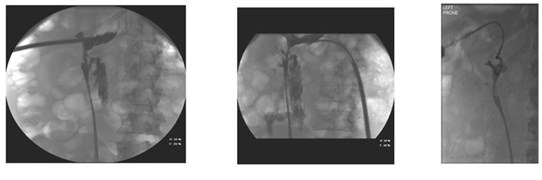
Figure 3: Pain on clamping nephrostomy with ensuing extravasation secondary
to distal obstruction followed by interval nephrostogram after antegrade stent.
Delayed bleeding with dropping haemoglobin and associated loin pain is usually secondary to a pseudoaneurysm or an arterio-venous fistula and typically occurs at around five days, but can happen anytime one to three weeks postoperatively. Once again renal angiography with super-selective embolisation is the solution.
Organ injury
Colonic perforation is a rare complication of PCNL, this is in part due to the preoperative planning of cases and the relative low incidence of the retro-renal colon (<7%) [30].
Access through the 10th intercostal space and anterior to the posterior axillary line is the main risk factor for adjacent organ injury. Colonic, splenic, and liver perforation are rare complications ranging from 0-0.4% in series with >1000 patients [31]. However, advanced age, horseshoe kidney and left-sided operations were deemed independent predictors of colonic injury during PCNL [32].
Sepsis
Despite antibiotics on induction of general anaesthetic, it is not unusual for complex stone patients to have a transient bacteraemia and subsequent pyrexia during the postoperative course. Urinary sepsis is the most common complication following PCNL [23,33] and ranges from 0.7-4%.
It is therefore crucial to have contemporary urine culture and sensitivities in the lead-up to the operation and to work closely with the microbiology team to adjust treatment algorithm dependent on the stone culture and subsequent clinical response. It is useful to send the intraoperative stone specimen for culture and sensitivity, as well as biochemistry, since this has the highest correlation to postoperative sepsis [13].
The duration of surgery and amount of irrigation are significant risk factors that contribute to postoperative fever [34]. Adequate drainage is particularly pertinent in such cases; the nephrostomy that is present should be left on free drainage without clamping and adequate targeted antibiotics prescribed.
Follow-up
The definition of ‘stone free’ varies considerably within the literature; inevitably this is also inextricably linked to the chosen follow-up modality. Whilst ultrasound follow-up is deemed acceptable in some cases, in other situations it is crucial to ensure stone-free status (complex matrix / struvite stones), to prevent growth and re-establishment of significant stone burden.
To this end a non-contrast CT KUB in the immediate postoperative period may help to risk stratify complex patients who may have a small amount of stone left and could benefit from a same admission relook through the established nephrostomy track.
Raman et al. charted the natural history of residual fragments after PCNL and found 67% of patients became symptomatic if the fragment was >4mm [35] – such patients may benefit from planned additional treatment rather than waiting for the stone to grow, or for an episode of ureteric colic.
Conclusion
PCNL is still considered as the ‘flagship’ operation in the endoluminal endourologist’s arsenal of operative techniques. However, despite the benefit of superior stone clearance rates, it comes with a higher risk profile. Although the percutaneous access into the pelvicalyceal system remains as the factor most at the forefront of successful stone clearance, the intra and postoperative approaches play an equally important part in determining a positive outcome. In this article, we have tried to outline some of the salient features in planning during PCNL, while emphasising that this is a team approach before, during and after the time that the patient is actually in the operating theatre for the procedure itself.
References
1. Fernström I, Johansson B. Percutaneous pyelolithotomy. A new extraction technique. Scand J Urol Nephrol 1976;10(3):257-9.
2. Helal M, Black T, Lockhart J, Figueroa TE. The Hickman peel-away sheath: alternative for pediatric percutaneous nephrolithotomy. J Endourol 1997;11(3):171-2.
3. Ruhayel Y, Tepeler A, Dabestani S, et al. Tract sizes in miniaturized percutaneous nephrolithotomy: a systematic review from the European Association of Urology Urolithiasis Guidelines Panel. Eur Urol 2017;72(2):220-35.
4. Huusmann S, Nagele U, Herrmann TR; on behalf of the Training and Research in Urological Surgery and Technology (T.R.U.S.T.) Group. Miniaturization of percutaneous nephrolithotomy: smaller, but better? Curr Opin Urol 2017;27(2):161-9.
5. Michel MS, Trojan L, Rassweiler JJ. Complications in percutaneous nephrolithotomy. Eur Urol 2007;51(4):899-906.
6. Canales B, Monga M. Surgical management of the calyceal diverticulum. Curr Opin Urol 2003;13(3):255-60.
7. Doré B, Conort P, Irani J, et al. [Percutaneous nephrolithotomy (PCNL) in subjects over the age of 70: a multicentre retrospective study of 210 cases]. Prog Urol 2004;14(6):1140-5.
8. Tefekli A, Kurtoglu H, Tepeler K, et al. Does the metabolic syndrome or its components affect the outcome of percutaneous nephrolithotomy? J Endourol 2008;22(1):35-40.
9. Burgher A, Beman M, Holtzman JL, Monga M. Progression of nephrolithiasis: long-term outcomes with observation of asymptomatic calculi. J Endourol 2004;18(6):534-9.
10. Singh M, Chapman R, Tresidder GC, Blandy J. The fate of the unoperated staghorn calculus. Br J Urol 1973;45(6):581-5.
11. Mak DK, Smith Y, Buchholz N, El-Husseiny T. What is better in percutaneous nephrolithotomy – Prone or supine? A systematic review. Arab J Urol 2016;14(2):101-7.
12. Patel RM, Okhunov Z, Clayman RV, Landman J. Prone versus supine percutaneous nephrolithotomy: what is your position? Curr Urol Rep 2017;18(4):26.
13. Kamphuis GM, Baard J, Westendarp M, de la Rosette JJ. Lessons learned from the CROES percutaneous nephrolithotomy global study. World J Urol 2015;33(2):223-33.
14. Sampaio FJ. Renal anatomy. Endourologic considerations. Urol Clin North Am 2000;27(4):585-607.
15. Osman M, Wendt-Nordahl G, Heger K, et al. Percutaneous nephrolithotomy with ultrasonography-guided renal access: experience from over 300 cases. BJU Int 2005;96(6):875-8.
16. Liu Q, Zhou L, Cai X, et al. Fluoroscopy versus ultrasound for image guidance during percutaneous nephrolithotomy: a systematic review and meta-analysis. Urolithiasis 2017;45(5):481-7.
17. Bayles A, Chitale S, Irving S, Burgess N. An audit of percutaneous nephrolithotomy in the United Kingdom. British Journal of Medical and Surgical Urology 2011;4(3):119-25.
18. Muslumanoglu AY, Tefekli A, Karadag MA, et al. Impact of percutaneous access point number and location on complication and success rates in percutaneous nephrolithotomy. Urol Int 2006;77(4):340-6.
19. Lu MH, Pu XY, Gao X, et al. A comparative study of clinical value of single B-mode ultrasound guidance and B-mode combined with color doppler ultrasound guidance in mini-invasive percutaneous nephrolithotomy to decrease hemorrhagic complications. Urology 2010;76(4):815-20.
20. Mariappan P, Loong CW. Midstream urine culture and sensitivity test is a poor predictor of infected urine proximal to the obstructing ureteral stone or infected stones: a prospective clinical study. J Urol 2004;171(6 Pt 1):2142-5.
21. Zhao PT, Hoenig DM, Smith AD, Okeke Z. A randomized controlled comparison of nephrostomy drainage vs ureteral stent following percutaneous nephrolithotomy using the Wisconsin Stone QOL. J Endourol 2016;30(12):1275-84.
22. Xun Y, Wang Q, Hu H, et al. Tubeless versus standard percutaneous nephrolithotomy: an update meta-analysis. BMC Urol 2017;17(1):102.
23. Armitage JN, Withington J, Van der Meulen J, et al. Percutaneous nephrolithotomy in England: practice and outcomes described in the Hospital Episode Statistics database. BJU Int 2014;113(5):777-82.
24. Opondo D, Tefekli A, Esen T, et al. CROES PCNL Study Group. Impact of case volumes on the outcomes of percutaneous nephrolithotomy. Eur Urol 2012;62(6):1181-7.
25. Withington JM, Charman SC, Armitage JN, et al. Hospital volume does not influence the safety of percutaneous nephrolithotomy in England: a population-based cohort study. J Endourol 2015;29(8):899-906.
26. Lee WJ, Smith AD, Cubelli V, et al. Complications of percutaneous nephrolithotomy. Am J Roentgenol 1987;148(1):177-80.
27. Wollin DA, Preminger GM. Percutaneous nephrolithotomy: complications and how to deal with them. Urolithiasis 2018;46(1):87-97.
28. Leavitt DA, Theckumparampil N, Moreira DM, et al. Continuing aspirin therapy during percutaneous nephrolithotomy: unsafe or under-utilized? J Endourol 2014;28(12):1399‑403.
29. BAUS. PCNL Outcomes Data.
www.baus.org.uk/patients/
surgical_outcomes/pcnl/about.aspx
30. Balasar M, Kandemir A, Poyraz N, et al. Incidence of retrorenal colon during percutaneous nephrolithotomy. Int Braz J Urol 2015;41(2):274-8.
31. de la Rosette J, Assimos D, Desai M, et al.; CROES PCNL Study Group. The Clinical Research Office of the Endourological Society Percutaneous Nephrolithotomy Global Study: indications, complications, and outcomes in 5803 patients. J Endourol 2011;25(1):11-17.
32. Wu P, Wang L, Wang K. Supine versus prone position in percutaneous nephrolithotomy for kidney calculi: a meta-analysis. Int Urol Nephrol 2011;43(1):67-77.
33. Vorrakitpokatorn P, Permtongchuchai K, Raksamani EO, Phettongkam A. Perioperative complications and risk factors of percutaneous nephrolithotomy. J Med Assoc Thai 2006;89(6):826-33.
34. Doğan HS, Sahin A, Cetinkaya Y, et al. Antibiotic prophylaxis in percutaneous nephrolithotomy: prospective study in 81 patients. J Endourol 2002;16(9):649-53.
35. Raman JD, Bagrodia A, Gupta A, et al. Natural history of residual fragments following percutaneous nephrostolithotomy. J Urol 2009;181(3):1163-8.
Declaration of competing interests: None declared.






