Introduction
Emphysematous pyelonephritis (EPN) is an acute, severe, necrotising, bacterial infection of the renal parenchyma and surrounding tissues, with gas in the renal parenchyma, collecting system or perinephric tissue. Although it is rare, it is potentially life threatening and early recognition and treatment is the key to reducing mortality. Pneumaturia secondary to a gas forming renal infection was first described by Kelly and MacCallum in 1898 [1] but the term emphysematous pyelonephrits was coined by Schultz and Korfein in 1962 [2].
The disease has previously been described as renal emphysema, pyelonephritis emphysematous and pneumonephritis. It has been suggested that emphysematous pyelitis, with gas in the collecting system only, should be viewed as a separate entity, due to a good prognosis with medical management, in comparison to emphysematous pyelonephritis which has a higher mortality.
Presentation
EPN should be suspected when a patient with multiple co-morbidities presents with severe sepsis and circulatory failure, combined with uncontrolled blood glucose. Other presenting symptoms include flank pain, pyrexia, vomiting, dysuria and pneumaturia. In some cases, crepitus in the flank and scrotum may be felt. Although gas may be seen on plain radiographs or ultrasound scan (USS), and ultrasound is a useful screening tool, the gold standard for imaging to determine the extent of the disease and plan intervention is a CT scan.
Table1.
Michaeli et al. (1984)
Based on spread of gas in plain radiographs and intravenous pyelogram:
-
Gas in the renal parenchyma or perinephric tissue.
-
Gas in the kidney and its surroundings.
-
Extension of gas through fascia, or bilateral disease.
Wan et al. (1996)
Based on CT findings:
-
Renal necrosis with either absence of fluid content on CT or the presence of streaky / mottled gas pattern demonstrated on plain radiograph or CT with lung window display.
-
Renal or perirenal fluid collection in association with bubbly or loculated gas pattern or gas in the collecting system. (Type 1 is more severe than type 2.)
Huang and Tseng (2000)
Based on CT:
-
Gas in the collecting system only.
-
Parenchymal gas only.
-
a) Extension of gas into perinephric space; b) Extension of gas into pararenal space.
-
EPN in solitary kidney or bilateral disease.
Aswathaman et al.(2008) [Modified from Huang and Tseng (2000)]
-
Gas confined to the renal parenchyma.
-
Gas extending to perinephric space and confined within the Gerota’s fascia.
-
Gas extending beyond Gerota’s fascia.
-
Gas involving both kidneys or gas in a solitary functioning kidney.
Classification systems for EPN are based on radiological findings (Table 1). There have been four classification systems described: Michaeli et al. 1984 [3], Wan et al. 1996 [4], Huang and Tseng 2000 [5], and Aswathaman et al. 2008 [6].
Pathogenesis
The most common reported causative organism is E. coli (61-97% of cases) [5-9]. The second most common is Klebsiella pneumonia (3-25.8%). Other organisms reported include Proteus mirabilis, Proteus vulgaris, Pseudamonas auerginosa, Group D streptococcus, coagulase negative staphylococcus and Citrobacter. The majority of patients have diabetes mellitus (up to 95% [4,10]). Other risk factors documented are drug or alcohol abuse, neurogenic bladder, anatomical anomalies, renal tract obstruction and immunosuppression. There is a higher incidence of EPN in females than males.
Prognosis
Early diagnosis and treatment is key. Treatment options in EPN have evolved from an aggressive surgical approach to a more conservative approach. Although historically, EPN has had a very high mortality with mortality rates up to 78% prior to the 1970s, this has been lower in more recent studies, improving to approximately 40-50% [7,8]. The best survival rates were Kapoor et al. in 2010 [11] who reported a survival rate of 87% with kidney salvaged in 67%. There appear to be some identifiable negative predictors of outcome including hypotension (BP<90mmHg) [10], altered mental state [11], severe hyponatraemia [11], high serum creatinine (>1.4mg/dL) [8], need for haemodialysis [6], thrombocytopaenia (platelets <60 000/mm3)[8].
“Treatment options in EPN have evolved from an aggressive surgical approach to a more conservative approach.”
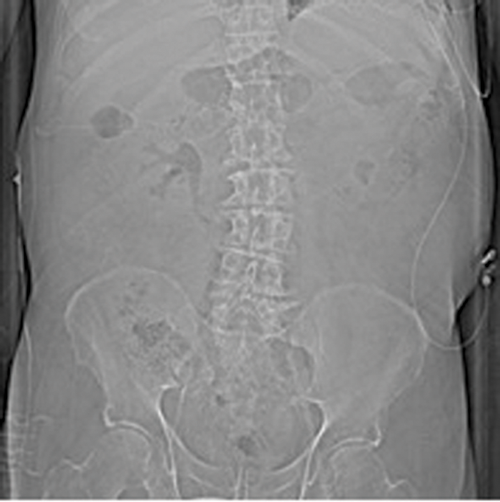
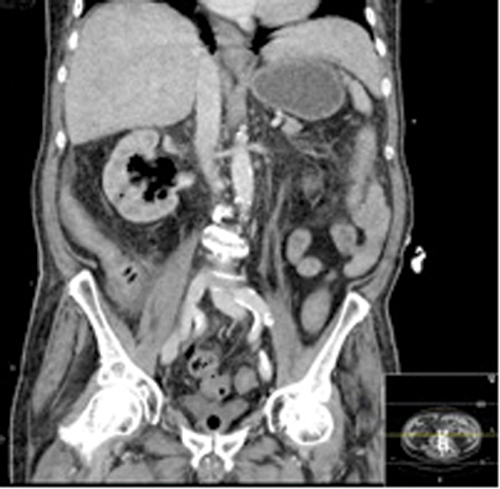
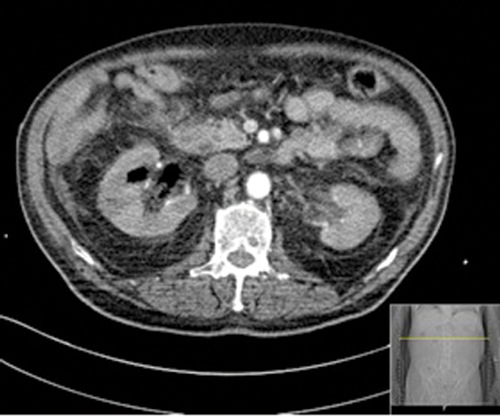
Figures 1: Patient A. Air in the collecting system extending into renal parenchyma.
Treatment and management
Following appropriate resuscitation, including intravenous antibiotics, fluid resuscitation and appropriate glycaemic control [7], further management should be taken on a case-by-case basis and the severity of the condition and the available services, such as interventional radiology, taken into consideration.
Antibiotic therapy
Gram-negative organisms are the most common causative organisms. Therapy should be therefore targeted initially as per local guidance, at gram negative organisms [12], prior to more specific sensitivities can be gained; allergy status permitting. Antibiotic therapy alone has been used and shown to be effective in 33.3-60% of cases [5,6]. Fifty percent (12/24) of cases were successfully treated with medical management in a review by Somani et al. [7]. There is a suggested role for long-term low-dose antibiotics post successful treatment of EPN with percutaneous nephrostomy and antibiotics [13], although the effectiveness of this therapy has not been quantified.
Percutaneous nephrostomy (PN)
Percutaneous nephrostomy for EPN was initially described in 1986 by Hudson [14]. Since then percutaneous nephrostomy and antibiotics has been suggested to be the initial therapy of choice for EPN. This nephron sparing technique has demonstrated variable success rates [7]. The success of PN has been directly related to the grade as suggested by Huang – the higher the grade, the increased chance of failure of PN from class I to IV. The suggestion is that percutaneous nephrostomy is indicated in class I, II, IV and IIIa and IIIb with <2 risk factors [5].
CT-guided drainage has been suggested to be favourable in comparison to USS [13] allowing assessment of the true anatomical relations of the loculations and air for the intended drainage. Multiple tracts have been used in the past with good success. Chen et al. [13] describe a series of PNs where up to three percutaneous tracts were used. These tracts were either sited initially or at intervals depending on the patient’s condition or interval CT scanning. Time to resolution with a PN has been variable and can take from as little as five days up to 12.6 weeks. It has been shown that the time to removal of drains where multiple tracts have been used is longer than a single tract. Interval CT scanning has suggested to be most effective at four to seven days.
The initial management of bilateral EPN has been suggested to be bilateral PN, due to the instability of patients [5] but this could also lead to a nephron-sparing approach.
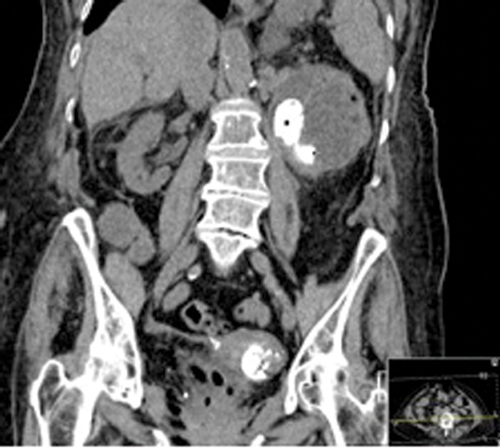
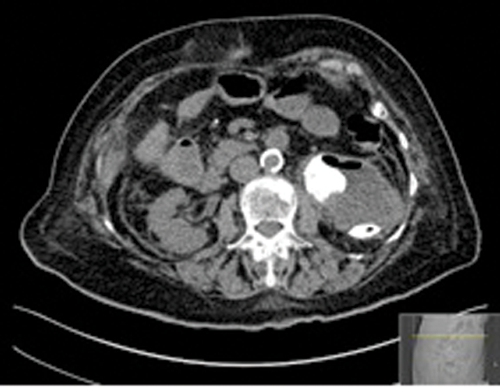
Figures 2: Patient B. Emphysematous pyelonephritis GM.
Laparoscopic nephrectomy and open nephrectomy
Huang and Tseng [5] suggested a clinical and radiological classification system (Table 1). This system indicates immediate nephrectomy in high-risk class 3a/3b. High-risk was defined as having ≥2 risk factors (thrombocytopenia, acute renal failure, disturbance of consciousness or shock). This classification system also suggested nephrectomy where initial management (PN and antibiotics) had failed. Early nephrectomy has been associated with an increased mortality in comparison to medical management and PN, 25% (16/64) to 13.5% (16/118), respectively [7].
The first laparoscopic nephrectomy for EPN was reported by Bauman in 2005 [15]. The length of stay in laparoscopic nephrectomy for EPN has been reported as shorter than both open nephrectomy and treatment with PN and antibiotics [16].
Delayed nephrectomy
When comparing delayed nephrectomy (initial nephrostomy and medical management) and percutaneous nephrostomy, it has been demonstrated that there is an overall lower mortality in the delayed nephrectomy group, 6.6% (1/15) compared to 13.5% (16/118), respectively [7].
In some cases a PN had completely drained the collection but the delayed nephrectomies were performed for non-functioning kidneys or staghorn calculi [7,13]. Elective nephrectomies have also been performed for prolonged sepsis and fever [7].
References
1. Kelly HA, MacCallum WG. Pneumaturia. JAMA 1898;31:375.
2. Schultz EH Jr, Klorfein EH. Emphysematous pyelonephritis. J Urol 1962;87:762-6.
3. Michaeli J, Mogle S, Perlberg S, et al. Emphysematous pyelonephtitis. J Urol 1984;131(2):203-7.
4. Wan YL, Lee TY, Bullard MJ, Tsai CC. Acute gas-producing bacterial renal infection: correlation between imaging indings and clinical outcome. Radiology 1996;198(2):433-8.
5. Huang JJ, Tseng CC. Emphysematous Pyelonephritis: Clinical radiological classification, management, prognosis and pathogenesis. Arch Intern Med 2000;160(6):797-805.
6. Aswathaman K, Gopakakrishnan G, Gnanaraj L, et al. Emphysematous pyelonephritis: outcome of conversative management. Urology 2008;71(6):1007-9.
7. Somani BK, Nabi G, Thorpe P, et al. Is percutaneous drainage the new gold standarad in the management of emphysematous pyelonephritis? Evidence from a systematic review. J Urol 2008;179(5):1844-9.
8. Wan YL, Lo S, Bullard MJ, et al. Predictors of outcome in emphysematous Pyelonephritis. J Urol 1998;159(2):369-73.
9. Tang HJ, Li CM, Yen MY, et al. Clinical characteristics of emphysematous pyelonephritis. J Microbiol Immunol Infect 2001;34(2):125-30.
10. Falgas ME, Alexopi VG, Giannopoulou KP, Siempos II. Risk factors for mortality in patients with emphysematous pyelonephritis: A meta-analysis. J Urol 2007;178(3):880-5.
11. Kapoor R, Muruganandham K, Gulia AK, et al. Predictive factors for mortality and need for nephrectomy in patients with emphysematous pyelonephritis. BJU int 2010;105(7);986-9.
12. Pontin AR, Barnes RD. Current management of emphysematous pyelonephritis. Nat Rev Urol 2009;6(5):272-9.
13. Chen MT, Huang CN, Chou YH, et al. Percutaneous drainage in the treatment of emphysematous pyelonephritis: 10-year experience. J Urol 1997;157:1569-73.
14. Hudson MA, Weyman PJ, Van der Vliet AH, Catalona WJ. Emphysematous pyelonephritis: successful management by percutaneous drainage. J Urol 1986;136:884.
15. Bauman N, Sabbagh R, Hanmiah R, Kapoor A. Laparoscopic nephrectomy for emphysematous pyelonephritis. Can J Urol 2005;12(4):2764.
16. Royle J, Williamson R, Strachan M, et al. Emphysematous pyelonephritis successfully treated with laparoscopic nephrectomy. Br J Med Surg Urol 2009;2(5):204-7.
TAKE HOME MESSAGE
-
In recent years mortality has improved
-
Focus shifting to salvage of the renal unit in appropriate cases
-
Delayed nephrectomy after initial medical management appears to be superior to emergency nephrectomy in acute severe EPN
Declaration of competing interests: None declared.