The first two case series that documented the association between urinary tract damage and recreational ketamine use were published 12 years ago [1,2]. Since then ketamine has maintained a controversial profile as an essential medication of expanding utility but with the potential for social misuse.
In the medical world it remains a useful anaesthetic with dissociative properties and is widely used amongst anaesthetists and emergency physicians. Its benefits include a short half-life, a lack of associated respiratory depression and administration through both intravenous and intramuscular routes. Recreationally, it is a drug with considerable addictive potential. Ketamine may be snorted, ingested, smoked or injected and can lead to derealisation, perceptual heightening and subjective euphoria.
Ketamine regulation and recreational use
In 2014, ketamine was reclassified from category C to B within the UK Misuse of Drugs Act due to “new evidence of chronic toxicity to the bladder” [3]. Statistics from the Home office note police seizure of ketamine increased by 30% in 2018 and that in 2019 use was recorded at 3% amongst young people (16-24 years old) up from 1.8% in 2014 and 0.9% in 2013 [4]. A similar growth in use was seen in Asia where, in China, 190,000 registered ketamine users were documented, of which 60% were under 25 years old. In Hong Kong, where ketamine has become the most commonly used psychotropic drug of abuse, ketamine remains regulated under Schedule I of the Dangerous Drugs Ordinance (chapter 134) and therefore shares the same status as cocaine, heroin and methamphetamine [5].
Since 2012, ketamine has been subject to three World Health Organization (WHO) reviews addressing its international control. Most recently, in 2016 the government of China proposed that it was placed in schedule I of the Convention on Psychotropic Substances, but WHO recommended against this change. It was concluded that the medical benefits of ketamine far outweigh its potential harm through recreational misuse and that stricter scheduling may negatively impact poorer health economies with limited access to alternative medications [6,7].
It is important to make a distinction between the dosing habits and subsequent side-effect profiles associated with safe medical ketamine prescribing (ranging from 1-4mg/kg for 5-10 minutes of sedation) versus that of sustained recreational use where individuals can routinely use as much as 15g in 24 hours.
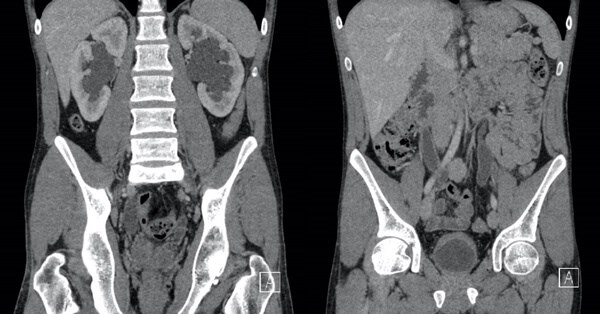
Figure 1: Coronal contrast CT images of a 23-year-old male patient with a prolonged history of recreational ketamine use, which shows bladder and ureteric wall thickening with hydroureteronephrosis.
Ketamine and the urinary tract
Ketamine uropathy (KU) describes the effects of ketamine on the urinary system. It is a misnomer to use ketamine cystitis or ketamine bladder syndrome as, whilst the bladder is often most severely affected, the whole urinary tract may be damaged. Recreational drug use should be asked about in patients with unexplained lower urinary symptoms or suprapubic pain. A positive history of recreational ketamine use and the absence of an alternative cause for symptoms such as infection, malignancy or stone disease would raise the suspicion of ketamine uropathy.
As a rule, patients present with severe suprapubic pain that can only be controlled with strong analgesia and with easy access to ketamine this can perpetuate their use. Cystoscopy may reveal a small capacity bladder, with contact bleeding and an angry, very inflamed and ulcerated macroscopic appearance that is confirmed on histology. Urothelial cells display atypia with a marked histological similarity to carcinoma in situ being described [8].
A 2019 Taiwanese study of 106 ketamine users noted a prevalence of lower urinary tract symptoms (LUTS) in 84%, commencing at a mean of 24 months after starting daily use. All symptom severity scores (overactive bladder symptom score, international prostate symptom score, interstitial cystitis symptom / pain index) correlated with duration of ketamine abuse. Their data suggested higher symptom scores where ketamine had been snorted when compared to those smoking a similar dose. There appears to be a dose dependent relationship between ketamine and symptom severity [9], a finding which is supported by in vivo research [10,11]. The largest study assessed symptoms in 1056 male users and noted a prevalence of 76%. Again, they found a significant correlation between higher age (>30 years), longer duration of use (>24 months) and co-use of other illicit drugs with symptom severity [12].
Sustained ketamine use results in a painful, contracted and fibrotic bladder, which leads to a loss of compliance, increased end-fill pressures and, in some, hydronephrosis. It is not known whether vesicoureteral reflux and urinary stasis or the antegrade flow of ketamine contaminated urine may cause ureteric inflammation, stricture and retroperitoneal fibrosis [13,14].
“A positive history of recreational ketamine use and the absence of an alternative cause for symptoms such as infection, malignancy or stone disease would raise the suspicion of ketamine uropathy”
Ketamine appears to be relatively ubiquitous in its cellular and receptor interactions making it difficult to specify a precise pathological mechanism for cystitis or other damage to the urinary tract. There certainly appears to be a combination of direct urothelial toxicity, neurogenic inflammation, immune mediated inflammation and cell apoptosis [13,15]. The severe pain experienced with the condition can be explained by nerve cell hyperplasia within the lamina propria as well as the occurrence of prominent nerve fascicle hyperplasia superficially, a finding not observed in other painful bladder conditions [16].
Ketamine may also negatively influence the tissue repair process by upregulating genes involved in extracellular matrix deposition. The excess of extracellular matrix promotes fibrosis within the detrusor and has been shown to develop in a dose dependent manner in rodent models. Evidence in animal models has also pointed towards ketamine interfering with key components of the calcium signalling pathway that is responsible for detrusor contraction, which may result in spontaneous or over-activity of the muscle [17].
Hydronephrosis in ketamine uropathy follows bladder fibrosis and contraction and occurs in 20-40% of patients with bilateral hydronephrosis being more common than unilateral. Ureteric wall thickening is commonly seen accompanying hydronephrosis however is not always present. Ureteric stricture, ureteric calculus, PUJ obstruction and renal papillary necrosis have all been reported as causes of hydronephrosis in KU. Urodynamics are effective at assessing the impairment to bladder function, however it is often a poorly tolerated investigation because of pain. Imaging of the upper urinary tracts is essential to exclude the potential of an obstructive nephropathy and it is the author’s preference to use ultrasound to exclude this in the first instance.
Nuclear imaging or contrast studies can be useful to characterise the impact and anatomy of upper tract obstruction if present. It is important to consider that the macroscopic severity of cystitis at cystoscopy cannot be used to predict the presence of upper tract involvement. Furthermore, ureteric inflammatory change and hydronephrosis have been reported within six months of previously normal imaging, suggesting that upper tract involvement and nephropathy can develop rapidly [18-20].
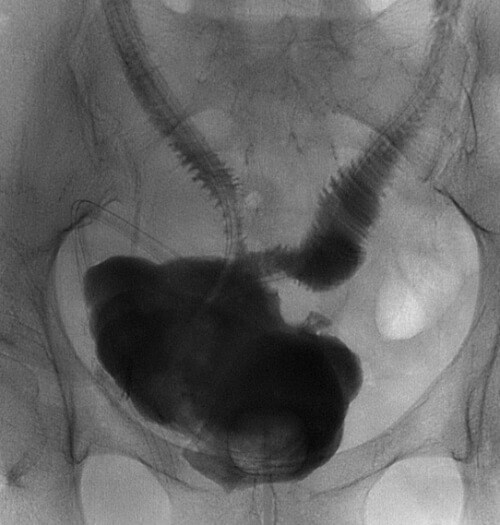
Figure 2: Cystogram of a 31-year-old female patient with a history of ketamine uropathy. The contrast study followed a multiple stage augmentation cystoplasty and bilateral ileal chute reconstructions. On the right the ileal chute extends from the lower pole renal calyx and on the left from the proximal ureter. The segments of bowel have then been anastomosed in a 'Wallace' fashion to the augmented bladder.
Management of ketamine uropathy
Whilst examples of treatment algorithms exist to manage the symptoms of ketamine uropathy, there is no universally agreed approach. Some recommendations share their management with guidelines for interstitial cystitis, which may have value. The inflammatory basis between the two conditions however differs and the potential for nephropathy in KU needs assessment and potential intervention. Stopping ketamine use is essential and given the severity of pain that patients experience, a well-tailored analgesic regime is vitally important. This is best developed with expertise from a specialist pain team with further support from addiction services and psychological counselling, if available, to prevent relapse.
The pelvic pain, urgency and frequency (PUF) questionnaire is validated in KU and has been used to monitor treatment response, as well as less commonly the ICSI / ICPI questionnaires. Hong et al. commented on a correlation between larger volume voids and lower PUF scores with increased duration since stopping ketamine [21]. Nonetheless, sustained cessation is obligatory as, with the exception of the insertion of a stent or nephrostomy due to an acutely obstructed kidney, any other urological intervention for KU is futile until at least six months of proven abstinence.
In Hong Kong, in order to engage the young ketamine using population, they have adopted an outreach model that works closely with anti-drugs social work services and allows patients with a history of ketamine use to access a urological consultation through their teacher, social worker or a self-referral hotline. This was developed with the primary aim of achieving high rates of early cessation and concentrating patient management to a single specialist centre. At this centre their four-tiered approach to management escalates from simple analgesia combined with anticholinergic medication, to opioid analgesia combined with neuropathic agents, such as pregabalin. Surgically, it progresses to minimally invasive interventions such as six-weekly topical instillations of sodium hyaluronate and as a last resort, invasive and reconstructive surgery if deemed necessary. Integral to this design is psychological support and therapy, alongside removal from predisposing social circumstances [21-23].
Likewise, in the UK, a similarly tiered system has been developed which compromises use of anticholinergics with or without B3 adrenoceptor agonists for presentations predominated by urinary storage symptoms, followed by a trial of Botulinum Toxin A detrusor injections. Other minimally invasive surgical interventions include cystodistension, urethral dilatation and intravesical sodium hyaluronate (Cystistat®). Where bladder pain is significant, which is very common, analgesic regimes are often tailored to patients and can involve non-steroidal anti-inflammatory drugs (NSAIDs), opioid analgesia as well as neuropathic agents, commonly amitriptyline. Pentosan polysulphate (Elmiron®) has also been employed as an oral agent. At each stage there is an emphasis on the use of adjunctive behavioral support measures and regular multidisciplinary team (MDT) discussion [14]. There is no clear evidence for any one strategy over another.
There appears to be correlation between a lack of treatment response to conservative measures and those who have used ketamine more extensively and where the degree of detrusor fibrosis therefore may be well established [23]. Whilst a moderate volume of data exists to demonstrate effective symptom reduction using the above pharmacological and minimally invasive surgical interventions, there is no large scale study to conclusively guide management [14,21-24]. Unfortunately, with many patients coming from poorer socioeconomic backgrounds, sometimes paired with a history of psychological ill-health, this can lead to high relapse rates and patients returning to ketamine use after apparent cessation. Reliable data are very difficult to gather.
Recent research into new therapeutic strategies has suggested N-acetyl-cysteine may protect against bladder fibrosis induced by ketamine. As a result, an improvement in the assessed urine storage parameters was seen when assessed in rodent models [25].
In 2016, Wu et al. were the first to propose a clinical staging of ketamine associated urinary tract dysfunction based on their experiences with 126 patients in Guangzhou, China [24]:
- Stage 1 is known as the inflammatory stimulation stage, characterised by normal upper tracts, renal function and lack of bladder contracture. They found this stage was responsive to bladder training and pharmacotherapy.
- Stage 2 is the initial bladder fibrosis stage, where patients demonstrate thickened bladder walls with reduced capacity, with occasionally involved upper tracts and preserved renal function. They suggest this stage can receive limited benefit with minimally invasive means such as hydrodistension and intravesical agents.
- Stage 3 represents patients with established fibrosis and bladder contracture, ureteric wall inflammation, hydronephrosis and decompensated renal function. In their series, these patients were managed with augmentation cystoplasty or urinary diversion with or without cystectomy. Patients in stages 2 or 3 with impaired drainage on renography were managed with ureteric stenting.
Whilst this model provides useful guidance, the authors acknowledged that it requires further validation.
Where patients have the equivalent of stage 3 ketamine uropathy, reconstructive surgery may offer improvement. Indications for surgical reconstruction include significant refractory lower urinary symptoms or pain, often with functional capacity <200ml and compliance pressures equal to or greater than 40cmH20. The presence of obstructive nephropathy is an absolute indication for intervention.
Such reconstructive options can include augmentation cystoplasty, cystectomy and neobladder formation or ileal conduit formation. Reconstruction or re-implantation of the ureters may also be required depending on upper tract involvement [14]. Bladder augmentation must be reserved for patients whose pain has resolved. The undertaking of invasive surgery in the context of late stage ketamine uropathy must however not be underestimated by either surgeon or patient. In the literature, of the four augmentation cystoplasties performed by Ng et al. there was a 100% ketamine relapse rate and of the 14 performed by Chung et al. 30% returned to ketamine use post-surgery [27,28]. It is therefore a common requirement amongst urological surgeons that patients have stopped ketamine and have not used in at least six months prior to potential restorative surgery. Due to this strict criterion, of the 319 patients with KU followed up by Yee et al. in Hong Kong, only one qualified to undergo augmentation cystoplasty [23].
Furthermore, the largest UK series of tertiary patients, who underwent restorative surgery due to KU, reported an unexpectedly high rate of postoperative complications. This included 14 patients, who underwent a combination of substitution cystoplasty or augmentation cystoplasty with or without mitrofanoff formation and ureteric interposition, of which 10 patients suffered complications. Sihra et al. hypothesised that the inflammatory effect of ketamine on the urinary tract and its surrounding tissues may interfere negatively with the surgical healing process, a factor which increases the risks associated with an already challenging surgery [14]. These elements highlight the multidisciplinary approach and care needed pre and postoperatively when treating patients affected by ketamine use.
The future
Going forward, there is a suggestion that ketamine misuse will continue to be a problem for global society, patients and in some cases urologists. In March 2019, intranasal esketamine was US FDA approved for the management of treatment resistant depression refractory to two oral antidepressant agents. Trial evidence was very promising and the breakthrough has been celebrated in psychiatric circles, however concerns are raised about whether sustained use of this medication could cause side-effects in the urinary tract. Whilst the proposed supervised doses are low compared to recreational amounts (for example 56 or 84mg OD twice weekly for four weeks), there remain questions regarding the optimal dose for maintenance as well as overall treatment duration.
Reassuringly, there have been no adverse urinary or other effects of esketamine reported during trials. It is also acknowledged that the symptoms of ketamine uropathy are a potential side-effect that those prescribing the medication must be aware of and will be monitoring for in the long term. A further concern might be the influence this new drug and its success may have on illicit ketamine use and manufacture. This includes the risk that members of the public may use it to self-medicate for their depression, particularly in countries where treatment is not licenced [30,31].
Conclusion
Since its first description, ketamine uropathy has become an independent urological entity with unique inflammatory and fibrosing effects on both the bladder and upper urinary tracts. With this in mind, it continues to be the subject of ongoing research into the aetiology of its damage and clinical learning into how it is optimally treated. The presence of a national database and establishing formalised clinical guidelines might aid urologists with more accurate and reliable patient data alongside treatment optimisation moving into the future.
TAKE HOME MESSAGE
-
It is important to ask about recreational drug use, specifically ketamine use, when assessing a patient with LUTS or bladder pain.
-
Upper tract imaging is essential to assess for potential hydronephrosis.
-
Cessation of ketamine use is vital to allow resolution of symptoms; many patients’ symptoms may resolve or significantly improve with this alone.
-
A multidisciplinary approach to patient management is important with regards to substance misuse, psychological assessment and choice of best urological therapy.
-
If indicated, reconstructive surgery is best performed in a tertiary setting and with confirmation that the patient has stopped ketamine for a minimum of six months without intention for future recreational use.
References
1. Shahani R, Streutker C, Dickson B, Stewart R. Ketamine-associated ulcerative cystitis: a new clinical entity. Urology 2007;69(5):810-12.
2. Chu P, Ma W, Wong S, et al. The destruction of the lower urinary tract by ketamine abuse: a new syndrome? BJU International 2008;102(11):1616-22.
3. Advisory Council on the Misuse of Drugs. Ketamine: a review of use and harm. 2013.
https://assets.publishing.service.gov.uk/
government/uploads/system/uploads/
attachment_data/file/264677/
ACMD_ketamine_report_dec13.pdf
[accessed 28 January 2020].
4. UK Home Office. Drug Misuse: Findings from the 2017/18 Crime Survey for England and Wales. 2018. https://assets.publishing.service.gov.uk/
government/uploads/system/uploads/
attachment_data/file/729249/
drug-misuse-2018-hosb1418.pdf
[accessed 28 January 2020].
5. Liao Y, Tang Y, Hao W. Ketamine and international regulations. The American Journal of Drug and Alcohol Abuse 2017;43(5):495-504.
6. INCB. Ketamine’s medical usage, abuse prevalence and control status. 2017
https://www.incb.org/documents/News/
Alerts/Alert_on_Control_of_Psychotropic
_Substances_December_2017.pdf
[accessed 28 January 2020].
7. WHO. Ketamine Update Review Report. 2014.
https://www.who.int/medicines/
areas/quality_safety/6_2_Update.pdf
[accessed 28 January 2020].
8. Baker SC, Stahlschmidt J, Oxley J, et al. Nerve hyperplasia: a unique feature of ketamine cystitis. Acta Neuropathol Commun 2013;1:64.
9. Li C, Wu S, Cha T, et al. A survey for ketamine abuse and its relation to the lower urinary tract symptoms in Taiwan. Scientific Reports 2019;9(1):7240.
10. Rajandram R, Yi Yap N, Ong TA, et al. Oral ketamine induced pathological changes of the urinary tract in a rat model. Malaysian J Pathol 2017;39(1):47-53.
11. Yeung L, Rudd JA, Lam WP, et al. Mice are prone to kidney pathology after prolonged ketamine addiction. Toxicology Letters 2009;191(2):275-8.
12. Yang S, Jang M, Lee K, et al. Sexual and bladder dysfunction in male ketamine abusers: a large-scale questionnaire study. PLOS ONE 2018;13(11):e0207927.
13. Misra S. Ketamine-associated bladder dysfunction – a review of the literature. Current Bladder Dysfunction Reports 2018;13(3):145-52.
14. Sihra N, Ockrim J, Wood D. The effects of recreational ketamine cystitis on urinary tract reconstruction – a surgical challenge. BJU International 2018;121(3):458-65.
15. Jhang J, Hsu Y, Kuo H. Possible pathophysiology of ketamine-related cystitis and associated treatment strategies. International Journal of Urology 2015;22(9):816-25.
16. Oxley JD, Cottrell AM, Adams S, Gillatt D. Ketamine cystitis as a mimic of carcinoma in situ. Histopathology 2009;55(6):705-8.
17. Shen C, Wang S, Wang S, et al. Ketamine‑induced bladder dysfunction is associated with extracellular matrix accumulation and impairment of calcium signaling in a mouse model. Molecular Medicine Reports 2019;19(4):2716-28.
18. Mason K, Cottrell A, Corrigan A, et al. Ketamine-associated lower urinary tract destruction: a new radiological challenge. Clinical Radiology 2010;65(10):795-800.
19. Huang LK, Wang JH, Shen SH, et al. Evaluation of the extent of ketamine-induced uropathy: the role of CT urography. Postgrad Med J 2014;90(1062):185-90.
20. Yee C, Teoh J, Lai P, et al. The risk of upper urinary tract involvement in patients with ketamine-associated uropathy. International Neurourology Journal 2017;21(2):128-32.
21. Hong YL, Yee CH, Tam YH, et al. Management of complications of ketamine abuse: 10 years' experience in Hong Kong. Hong Kong Med J 2018;24(2):175-81.
22. Tam YH, Ng CF, Pang KK, et al. One-stop clinic for ketamine-associated uropathy: report on service delivery model, patients' characteristics and non-invasive investigations at baseline by a cross-sectional study in a prospective cohort of 318 teenagers and young adults. BJU Int 2014;114(5):754-60.
23. Yee CH, Lai PT, Lee WM, et al. Clinical outcome of a prospective case series of patients with ketamine cystitis who underwent standardized treatment protocol. Urology 2015;86(2):236-43.
24. Wu P, Wang Q, Huang Z, et al. Clinical staging of ketamine-associated urinary dysfunction: a strategy for assessment and treatment. World J Urol 2016;34(9):1329-36.
25. Men E, Tsao W, Tang S, et al. Intravesical hyaluronic acid treatment for ketamine-associated cystitis: preliminary results. Neurological Science 2015;26(3):176-9.
26. Ryu C, Shin J, Yu H, et al. N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Scientific Reports 2019;9:8134.
27. Ng CF, Chiu PK, Li ML, et al. Clinical outcomes of augmentation cystoplasty in patients suffering from ketamine-related bladder contractures. Int Urol Nephrol 2013;45(5):1245-51.
28. Chung SD, Wang CC, Kuo HC. Augmentation enterocystoplasty is effective in relieving refractory ketamine-related bladder pain. Neurourol Urodyn 2014;33(8):1207-11.
29. Swainson J, Thomas R, Archer S, et al. Esketamine for treatment resistant depression. Expert Review of Neurotherapeutics 2019;19(10):899-911.
30. Jauhar S, Morrison P. Esketamine for treatment resistant depression. BMJ 2019;366:8215.
31. Psychiatrists face challenges in creating Spravato practices. Clinical Psychiatry News 2019. https://www.mdedge.com/psychiatry/
article/205275/depression/psychiatrists
-face-challenges-creating-spravato
-practices/page/0/2
[accessed 28 January 2020].
Declaration of competing interests: None declared.