Clinical benign prostatic hyperplasia (BPH) impacts on the quality of life of many men. It is intimately related to ageing, but exact calculations of its prevalence remain difficult since an accurate clinical definition still eludes us. Histological BPH has been shown to only occur in men over 30 and prevalence peaks in the ninth decade at 88% [1]. But since prostatic enlargement does not correlate directly to symptoms, clinical BPH has been harder to characterise. In the absence of a clear delineation of the disease, progression is defined not by a move from disease-free to diseased status but rather by deterioration in a number of physiological variables.
The most commonly used markers are decrease in maximum flow rate, increase in residual volume, increase in prostate size and deterioration in symptom score. Given that BPH / lower urinary tract symptoms (LUTS) cause such a spectrum of symptoms and severity, it is vital to tailor the management utilising conservative, medical and surgical treatment strategies.
Alpha-antagonists and five-alpha reductase inhibitors constitute the first-line in medical treatment, supported by several large randomised controlled trials (RCT) confirming their short and long-term efficacy in reducing LUTS. However surgery remains the only option for patients who are refractory to medical treatment. Whether the use of these drugs simply delays the inevitable need for surgery in certain patients is still a matter for debate, however the need for minimally invasive options in our ageing society remains strong and is highlighted by the continual plethora of novel techniques and technology that are made available to the budding urologist.
Transurethral resection of the prostate
Transurethral resection of the prostate (TURP) has been the mainstay of surgical treatment since its introduction in 1932 and remains the standard against which new techniques are measured. It has remained the reference standard because of the extensive, long-term data that supports it as an efficient, cost-effective and relatively durable surgical procedure. One of the reasons for the considerable research and energy expended in the search for alternate surgical treatments is the well-recognised morbidity that remains associated with TURP together with the growing number of minimally invasive and office-based procedures.
Developments in technology and techniques over the last decade have had a large impact on reducing complications. Intraoperative bleeding has been a significant problem with mean transfusion rates of 8.6% reported in an early series [2]. Through the introduction of new technology, such as the microprocessor controlled monopolar generator and coagulating intermittent cutting, and better operative techniques [3], the transfusion rate in contemporary studies has fallen to between 2% and 4.5% [4,5]. Likewise the incidence of clinical TUR syndrome has dropped from >2% to <1% with modern technical adaptations such as low pressure irrigation, improved irrigating fluids and again improved technique. Bipolar technology should make dilutional hyponatraemia even less common. The major advantage of transurethral resection remains the reproducible results with contemporary RCT meta-analyses demonstrating improvements in International Prostate Symptom Score (IPSS) of around 70% [6] and greater than 100% increases in peak flow rates. Urodynamic studies also confirm that TURP effectively de-obstructs the bladder outlet (and is only exceeded by open prostatectomy [2]).
The mean preoperative prostate volume has increased over the last decade despite the widespread use of medical therapies as first-line treatments. Nonetheless, while mean resected weights have risen, operative times have fallen, providing support for the increasing efficiency of TURP [7]. And although newer therapies may boast low morbidity and complications, the robust outcomes of a transurethral resection are yet to be beaten by any other technique. Its increasing safety and efficiency means that it continues to play a central role in the management of BPH.
Interstitial laser coagulation
Even though there are only two basic mechanisms by which LASERs (light amplification by stimulated emission of radiation) are used in the treatment of BPH, a confusing array of terms and technologies has been created around them. Not all lasers are equal!
Lasers can be used to either coagulate or vaporise tissue. Coagulation occurs when tissues are heated to below the vaporisation / boiling point but above that necessary for protein denaturation. The neodymium / yttrium-aluminium garnet (Nd:YAG) laser has a relatively long wavelength of 1,064nm. Given that the energy delivered by a laser is inversely proportional to the wavelength (the Planck relation), the laser delivers a low energy density and consequently lower temperatures within the tissues. This leads to coagulative necrosis and delayed de-bulking of the tissues. Laser coagulation was first achieved through visual laser ablation of the prostate (VLAP). A non-contact Nd:YAG laser is used to coagulate the prostatic and prostatic-urethral tissue. Although refinements have been made, the major drawback is severe irritative symptoms and urinary obstruction. These symptoms, caused by the sloughing of prostatic tissues, can last for months.
To overcome this, interstitial laser coagulation (ILC) was introduced in 1993 [8]. A Nd:YAG laser fibre is introduced into the prostatic tissue transurethrally under endoscopic guidance. Tissues are heated to 85oC and in the following months prostatic volume is reduced through the ensuing coagulative necrosis. ILC has a number of advantages. Operative morbidity and blood loss are almost non-existent and it can be performed under local anaesthesia. However the major drawbacks have been prolonged catheterisation time, dysuria, high postoperative infection rates and poor long-term durability. Reoperation rates have been shown to be up to 50% at five years [9]. Apart from a few areas, possibly with some influence from local remuneration systems, ILC has been superseded by newer laser techniques. We describe the commonest ones here.
GreenLight™ laser vaporisation of the prostate
Unlike coagulation, vaporisation of tissues requires tissues to be heated to above their vaporisation point. This requires a far higher energy density within the tissues, which is achieved through the modification of the Nd:YAG laser. A number of lasers have been developed which will vaporise tissue but the best studied and most widely used is the GreenLight™ (52nm) vaporisation. Initially, potassium-titanyl-phosphate (KTP) crystals were used to halve the wavelength of the Nd:YAG laser with a number of beneficial effects. Not only is the energy delivered increased but also the optical interaction of the laser is greatly improved. The KTP laser’s shorter wavelength gives green rather than infrared light, which is strongly absorbed by (red) haemoglobin and other tissue but is easily transmitted through aqueous irrigants. Increased absorption by haemoglobin leads to a much shallower depth of absorption (≈800µm) resulting in heat concentration within the tissues. Lysis and vaporisation of the tissue occurs with only a 2mm rim of coagulation. In addition there is little collagenous scar formation as seen in Nd:YAG coagulation. The overall effect is highly efficient vaporisation producing a non-contracted and smooth cavity that persists after healing [10].
Initially the KTP laser was used only as an adjunction in Nd:YAG laser coagulation to vaporise the treated tissues and reduce side-effects from sloughing. However, the benefits of pure vaporisation were soon realised and Malek et al. introduced photoselective vaporisation of the prostate (PVP) in 2000 using a 60W laser [10]. Since then there have been a number of developments, increasing power, efficiency and consequently both technique and speed of vaporisation. Power output has been boosted, originally to 80W, and then with lithium triborate (LBO) replacing KTP crystals, to 120W. The delivery systems themselves have been significantly improved. With ‘pulsing technology’, the laser undergoes high frequency modulation producing a succession of micropulses rather than a continuous wave increasing the power output further.
The most recent development has been the introduction of the GreenLight XPS™ MoXy™ laser fibre with a maximum power output of 180W. There has been a high uptake of PVP by urologists internationally, not least because of its short learning curve and safety in anti-coagulated patients. Although there are now a number of randomised controlled trials and meta-analyses to support its use as compared to TURP, long-term follow-up data is not available and the majority of data beyond two years still relates to the 80W KTP system. In general PVP has been found to be a safe and viable alternative to TURP. Outcomes (improvement in IPSS, Qmax, post void residuals) are similar to those of TURP, and PVP has been shown to have a strong safety profile (Figure 1).
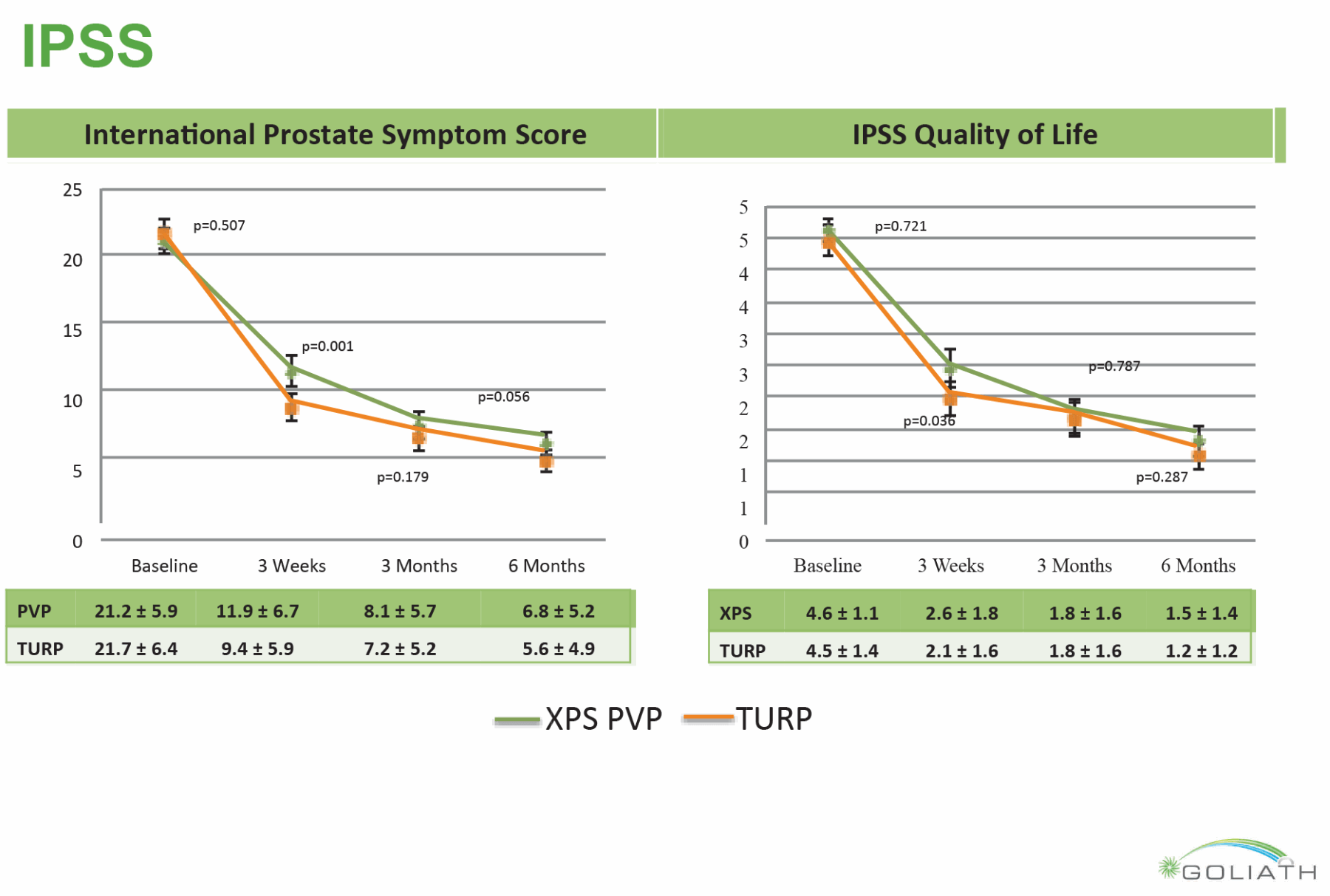
Figure 1: Qmax and Post Void Residual Results from The GOLIATH Study (Reprinted from European Urology, Epub ahead of print, Bachmann A, Tubaro A, Barber N, et al. 180-W XPS GreenLight Laser Vaporisation Versus Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: 6-Month Safety and Efficacy Results of a European Multi centre Randomised Trial—The GOLIATH Study. Copyright 2013, with permission from Elsevier.)
There is no risk of TUR syndrome, significantly lower blood loss and transfusion rates and low risk of capsular perforation [11]. Catheterisation times and hospital stays are also significantly shorter with one RCT even reporting 32% patients being catheter-free immediately postoperatively [12].
A number of studies, none randomised, have shown excellent safety and very low rates of significant bleeding even in patients with large prostates on continuing anticoagulation.
One of the disadvantages with early PVP was a significant re-intervention rate (between 8.9% [13] and 14.8% [14] at five years) in early studies. These were predominantly due to recurrent adenoma, bladder neck stricture and urethral strictures although the rates are comparable to many TURP series. More recent studies have used greater energy than the initial studies using the 80W machine, and early data suggest a lower re-intervention risk. Treatment of larger prostates (>80ml) is also controversial with early data showing high re-intervention rates, which may be due to some early studies having inadequate technique [11]. The 180W GreenLight XPS™ system is yet to be fully evaluated but its greater efficiency and vaporising speed have been shown in a large multicentre RCT to give results indistinguishable from TURP with lower complications and bleeding [15].
A controversial area is that of retrograde ejaculation (or dry orgasm) and erectile function. A number of authors have argued that PVP has lower rates of dry orgasm than other surgical de-bulking techniques [16]. Erectile function is more difficult to assess, there is evidence to suggest occasional reduction in erectile function scores but these are not seen in meta-analyses. As with most studies of LUTS/BPH surgery, the data on sexual side-effects is poorly presented, but in the recent GOLIATH study, they were minimal except for high rates of dry orgasm [17]. The International GreenLight Laser Users Group (IGLU) group has shown modification of GreenLight™ laser can bring dry orgasm rates below 20% with no evident change in the symptom outcome [18].
Figure 2: Transrectal ultrasound pre and post GreenLight vaporisation.
Holmium laser vaporisation and enucleation
The holmium: yttrium-aluminium-garnet (Ho:YAG) laser has a number of features that make it a valuable tool for the modern urologist. Its pulsed wavelength of 2140nm is strongly absorbed by water (making it retina safe). The high water content of prostatic tissue, together with saline irrigation, produces a shallow penetration depth, excellent haemostasis [19] and minimal dispersion of energy to surrounding tissues. It also produces little char effect and direct contact with the tissues allows for precise dissection. Likewise the superheating of water within calculi, also allows for excellent stone fragmentation. The Ho:YAG laser as a lithotrite in fact heralded its introduction to urology in 1998.
Since it was first introduced as a treatment for BPH in 1995 by Chun [20] and Gilling [21], a variety of techniques have been deployed evolving from ablation to resection and, most recently, enucleation. Holmium ablation of the prostate (HoLAP) was first reported in 1994; however the data evaluating its performance is sparse. In a single centre RCT that compared it against TURP, 60W and 80W HoLAP produced equivocal flow rate and symptom score improvement at 6, 12 and 18 months. The one advantage of a marginally reduced catheterisation time was offset by both a longer operative time and a smaller reduction in the prostatic volume [22]. Likewise, when compared against PVP, outcomes were again equivocal and operating time 1.5 times longer despite use of the more powerful 100W laser [23].
Holmium laser resection of the prostate(HoLRP), in which the vaporisation is used to cut sections of the prostate like the traditional TURP, was first introduced in 1995 [24]. Since it has now been superseded by HoLEP, no recent RCT or systematic reviews have been undertaken. A meta-analysis by Tooher et al. showed that HoLEP did result in a greater mean difference in the Qmax whilst symptom score improvement and morbidity markers were again comparable [25]. Few centres now seem to be using HoLAP or HoLRP except as possible stages in learning enucleation.
The introduction of the endoscopic tissue morcellator prompted the final cycle of the evolution of the holmium laser in the treatment in BPH, allowing anatomical holmium laser enucleation of the prostate (HoLEP). In this endoscopic equivalent of open prostatectomy, the holmium laser bare tip fibre is used to create a plane between the prostate and surgical capsule (the compressed peripheral zone and anterior fibromuscular stroma). The lobes are then removed individually and broken up with the morcellator. Despite relatively few RCTs, HoLEP is arguably the most rigorously analysed endoscopic laser technique. A number of meta-analyses have been performed against TURP which demonstrate a similar therapeutic efficacy to TUR whilst catheterisation time, hospital stay and blood loss were all improved [26,27]. Operation times were greater with HoLEP, however this may be partially due to the larger volume of prostatic tissue removed, for when the rate of tissue removal is compared, HoLEP is significantly more efficient.
A major drawback of the use of the Ho:YAG may be its technical complexity. Experience is the most important factor in overall rate of complications [27]. In training centres, trainees must perform at least 20-30 moderately sized cases under expert guidance before they can reproduce consistent, good results. Although arguably more complicated to learn than vaporisation techniques, the early standardisation of HoLEP technique has allowed direct comparability of results, which is a problem in many of the earlier GreenLight™ papers.
Other lasers
A number of other lasers (e.g. EVOLVE® diode laser, VersaPulse® thulium laser) have been developed for both vaporisation, enucleation and laser resection of BPH. Few have more than a couple of papers supporting their use. In nearly all cases one can state that if a large cavity is produced in the prostate a patient will void well. This is as true for patients in retention as for elective surgery. One problem in using new lasers is safety – the tissue interaction of any given wavelength will vary according to the power and delivery technique. It therefore seems to the authors a mandatory requirement that new prostate lasers have a basic assessment of efficiency, vaporisation depth and coagulation depth before being used in patients for the first time.
The future
Lasers can be expensive, but almost all studies of the commonly used lasers show that in well trained hands they can deliver the same results as conventional surgery with lower side-effects and much shorter hospitalisation. The inexorable move to outpatient surgery may drive their adoption, even if patient safety does not. But there is not one ‘ideal’ operation for every patient and every prostate: prostate volume, patient co-morbidity, concomitant anticoagulation and desire for preserving sexual function should now be considered in every individual’s case, and so should locally available expertise. We believe that surgical lasers provide great advantages, particularly in large prostates and high-risk patients, and offer the tantalising prospect of tailoring surgery to the individual. How we find the evidence to support ‘bespoke’ surgery, and whether high-risk patients should be managed in BPH centres of excellence with access to multiple technologies, are debates for another time.
References
1. Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984;132(3):474-9.
2. Madersbacher S, Marberger M. Is transurethral resection of the prostate still justified? BJU Int 1999;83(3):227-37.
3. Haupt G, Pannek J, Benkert S, et al. Transurethral resection of the prostate with microprocessor controlled electrosurgical unit. J Urol 1997;158(2):497–501.
4. Ahyai SA, Gilling P, Kaplan SA, et al. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur Urol 2010;58(3):384–97.
5. Rassweiler J, Teber D, Kuntz R, Hofmann R. Complications of transurethral resection of the prostate (TURP)--incidence, management, and prevention. Eur Urol 2006;50(5):969–79, discussion 980.
6. Oelke M, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64(1):118–40.
7. Mayer EK, Kroeze SGC, Chopra S, et al. Examining the “gold standard”: a comparative critical analysis of three consecutive decades of monopolar transurethral resection of the prostate (TURP) outcomes. BJU Int 2012;110(11):1595–601.
8. McNicholas TA, Steger AC, Bown SG. Interstitial laser coagulation of the prostate. An experimental study. Br J Urol 1993;71(4):439–44.
9. Daehlin L, Frugård J. Interstitial laser coagulation in the management of lower urinary tract symptoms suggestive of bladder outlet obstruction from benign prostatic hyperplasia: long-term follow-up. BJU Int 2007;100(1):89–93.
10. Malek RS, Kuntzman RS, Barrett DM. High power potassium-titanyl-phosphate laser vaporization prostatectomy. J Urol 2000;163(6):1730–3.
11. Teng J, Zhang D, Li Y, et al. Photoselective vaporization with the green light laser vs transurethral resection of the prostate for treating benign prostate hyperplasia: a systematic review and meta-analysis. BJU Int 2013;111(2):312–23.
12. Te AE, Malloy TR, Stein BS, et al. Photoselective vaporization of the prostate for the treatment of benign prostatic hyperplasia: 12-month results from the first United States multicenter prospective trial. J Urol 2004;172(4 Pt 1):1404–8.
13. Hai MA. Photoselective vaporization of prostate: five-year outcomes of entire clinic patient population. Urology 2009;73(4):807–10.
14. Ruszat R, Seitz M, Wyler SF, et al. GreenLight laser vaporization of the prostate: single-center experience and long-term results after 500 procedures. Eur Urol 2008;54(4):893–901.
15. Bachmann A, Tubaro A, Barber N, et al. A Prospective Multicenter Randomized Study Comparing GreenLight XPS™ Vaporization and Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Hyperplasia (GOLIATH). J Urol 2013;189(4).
16. Metcalfe C, Poon KS. Long-term results of surgical techniques and procedures in men with benign prostatic hyperplasia. Curr Urol Rep 2011;12(4):265–73.
17. Bachmann A, Tubaro A, Barber N, et al. 180-W XPS GreenLight Laser Vaporisation Versus Transurethral Resection of the Prostate for the Treatment of Benign Prostatic Obstruction: 6-Month Safety and Efficacy Results of a European Multi centre Randomised Trial—The GOLIATH Study. Eur Urol 2013; [Epub ahead of print].
18. Talab SS, Santiago-Lastra YA, Bachmann A, et al. The Impact Of Ejaculation Preserving Photo-Selective Vaporization of the Prostate (EP-PVP) on Lower Urinary Tract Symptoms and Ejaculatory Function: Results of a Multicentre Study. J Urol 2013;189(4).
19. van Rij S, Gilling PJ. In 2013, Holmium Laser Enucleation of the Prostate (HoLEP) May Be the New “Gold Standard.” Curr Urol Rep 2012;13(6):427-32.
20. Chun SS, Razvi HA, Denstedt JD. Laser prostatectomy with the holmium: YAG laser. Techniques in Urology. 1995;1(4):217-21.
21. Gilling PJ, Cass CB, Malcolm AR, Fraundorfer MR. Combination holmium and Nd: YAG laser ablation of the prostate: initial clinical experience. J Endourol 1995;9(2):151-3.
22. Mottet N, Anidjar M, Bourdon O, et al. Randomized comparison of transurethral electroresection and holmium: YAG laser vaporization for symptomatic benign prostatic hyperplasia. J Endourol 1999;13(2):127–30.
23. Elmansy HM, Elzayat E, Elhilali MM. Holmium laser ablation versus photoselective vaporization of prostate less than 60 cc: long-term results of a randomized trial. J Urol 2010;184(5):2023–8.
24. Gilling PJ, Cass CB, Cresswell MD, Fraundorfer MR. Holmium laser resection of the prostate: preliminary results of a new method for the treatment of benign prostatic hyperplasia. Urology 1996;47(1):48–51.
25. Tooher R, Sutherland P, Costello A, et al. A systematic review of holmium laser prostatectomy for benign prostatic hyperplasia. J Urol 2004;171(5):1773-81.
26. Tan A, Liao C, Mo Z, Cao Y. Meta-analysis of holmium laser enucleation versus transurethral resection of the prostate for symptomatic prostatic obstruction. Br J Surg 2007;94(10):1201–8.
27. Lourenco T, Pickard R, Vale L, et al. Alternative approaches to endoscopic ablation for benign enlargement of the prostate: systematic review of randomised controlled trials. BMJ 2008;337:a449.
28. Elzayat EA, Elhilali MM. Holmium laser enucleation of the prostate (HoLEP): long-term results, reoperation rate, and possible impact of the learning curve. Eur Urol 2007;52(5):1465–71.
Declaration of competing interests: None declared.