All UK urologists, unless they have been on a 10-year silent retreat, are by now aware of the controversy surrounding surgical use of mesh in general and urological / urogynaecological use of mesh for the surgical treatment of stress urinary incontinence (SUI) and pelvic organ prolapse (POP) in particular.
The majority (but not all) of UK urologists are also no doubt aware of the current ‘High Vigilance Pause’ on the use of mesh surgery for POP and SUI in England [1]. This article attempts to demystify the background to this current pause and the likely future direction of SUI and POP and related surgery.
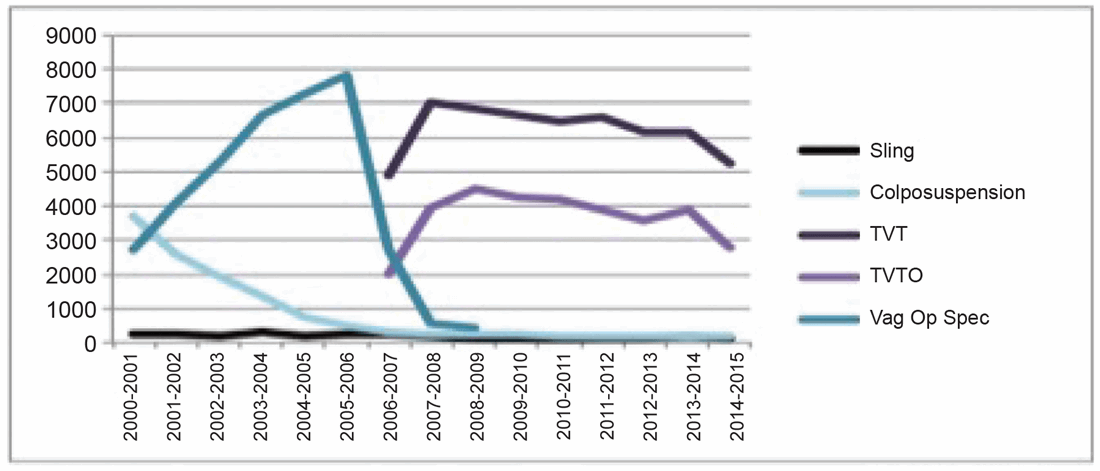
Figure 1: SUI Surgery NHS England 2000-2015.
FDA notification
Following Ulmsten’s 1998 publication on the excellent 12-month continence outcomes of a multicentre study of TVT for the treatment of SUI the technique went ‘viral’, becoming the most common surgical treatment for SUI worldwide by 2005 [2,3] (Figure 1). As the use of mesh for SUI and POP surgeries gained popularity documented complications for POP mesh surgery began to increase, prompting a Public Health Notification (PHN) by the US Food and Drug Administration (FDA) in 2008 [4]. This PHN targeted vaginal mesh for POP only and urged surgeons to inform patients of the potential complications. In 2014 the FDA adjusted its classification of transvaginal mesh from moderate to high risk [5].
NHS Scotland Independent Review
Following the FDA PHN an increasing number of UK patients came forward reporting significant complications following mesh surgery for POP and SUI. In June 2014 the Scottish Health Minister requested suspension of mesh surgery for SUI and POP in Scotland due to safety concerns and instigated an Independent Review. The Scottish Independent Mesh Review reported in 2016 with seven recommendations [6]. These were:
- Robust clinical governance around the decision to treat via multidisciplinary team meetings (MDT), audit and review.
- Evidence of (1) as part of mandatory appraisal and revalidation.
- Informed consent with a mandatory SUI patient information leaflet (PIL) and consent form and mandated development of a similar POP PIL.
- Research on long-term outcomes and quality of life in women having mesh surgery for SUI and POP to be prioritised.
- Improved coding for Hospital Episode Statistics (HES) and improved entry to professional databases.
- Concerns expressed regarding obturator c.f. suprapubic mesh for SUI – and obturator route not recommended unless specifically indicated.
- Similar concerns expressed regarding mesh for POP.
NHS England Mesh Oversight Group Report
A Mesh Oversight Group was established by NHS England in 2014 to review the current state of mesh surgery in England and provide oversight into:
- Clinical quality of care of women with SUI and POP.
- Data and information to support clinicians and women around decisions about POP and SUI surgery.
- The consent process.
The group was composed of representatives from: British Association of Urological Surgeons (BAUS), British Society of Urogynaecology (BSUG), Department of Health (DoH), Medicines and Healthcare products Regulatory Agency (MHRA), National Institute for Health & Care Excellence (NICE), Royal College of Obstetricians and Gynaecologists (RCOG), NHS England, Association of British HealthTech Industries, Meshies United, RCOG Women’s Network, TVT Messed up Mesh and independent patient members. In their final report released in July 2017 [7] they recommended that:
- Trust appraisal is used to ensure surgeons are appropriately trained and current in their practice; adhere to clinical guidance; comply with national data requirements; and report complications.
- NICE to produce a Clinical Guideline that describes, holistically, care for women with POP.
- NICE to review the current Clinical Guideline for Urinary Incontinence (CG171).
- NICE to review and create guidance on the management of complications arising from surgery for SUI and POP.
- A nurse helpline service for mesh-injured women to be established.
- GP awareness of treatment options for SUI and POP to be improved.
- Reporting adverse incidents to MHRA including reporting retrospectively to be encouraged.
- New OPCS codes should be developed to reflect complications.
- A one-off information gathering exercise on patient outcomes.
- Establishment of a registry for all mesh procedures.
- Consistent information should be given to patients on mesh procedures for treatment of SUI and POP through the use of PILs developed in line with national guidance in collaboration with clinicians, professional bodies and patient support groups.
- Good practice in obtaining legally informed consent to be followed.
- The use of unified PILs by all UK urologists and urogynaecologists ratified by the Royal College of Surgeons (RCS), BAUS, RCOG and BSUG.
- RCS, BAUS, RCOG and BSUG to regularly update these unified PILs.
- The relevant national NHS service logo to be appended to the unified PILs.
- RCS, BAUS, RCOG and BSUG to append their logos to the unified PILs. [8].
However, this report failed to bring clarity on the actual risk of mesh complications and their severity or the number of those affected. Furthermore, patient opinion and testimony were not a major determinant to the group’s final statement, an ongoing demand from the Sling the Mesh campaign group, who did not take part in this review.
“A government funded third party review of the outcomes of all women during a set time period having surgical treatment for SUI (mesh and non mesh) is what is actually required to delineate the magnitude of the issue”
NICE Guidance
In December 2017 NICE published their recommendation that mesh in the setting of POP surgery should be used only in the context of research due to inadequate long-term efficacy data [9]. An updated guidance for the management of urinary incontinence and pelvic organ prolapse in women (NG123) was published on 2 April 2019 [10].
This guidance suggests strict mandatory requirements prior to any surgery for SUI or POP. These include having mandatory local MDT discussion for women:
i) Contemplating surgery for primary SUI, overactive bladder (OAB) and POP.
ii) With primary SUI, OAB or POP where input from a wider range of health professionals is needed.
The core local MDT should be:
a) Two consultants with expertise in managing UI and / or POP in women.
b) A urogynaecology, urology or continence nurse specialist.
c) A pelvic floor physiotherapist.
The local MDT MUST work within an established clinical network with a regional MDT. Mandatory regional MDT discussion is suggested for women:
i) Contemplating surgery for recurrent SUI and / or recurrent urge urinary incontinence (UUI).
ii) Contemplating surgery for recurrent same site POP.
iii) With primary SUI or POP whose preferred treatment option is not available locally.
iv) With primary or recurrent SUI, UUI or POP who have associated bowel dysfunction.
v) Contemplating vaginal mesh for POP.
vi) With mesh complications or with unexplained symptoms following mesh SUI or POP surgery.
vii) Considering surgery for their SUI, UUI or POP who have not as yet completed their families.
The core regional MDT should be:
a) A subspecialist in urogynaecology.
b) A urologist with expertise in female urology.
c) A urogynaecology, urology or continence specialist nurse.
d) A pelvic floor specialist physiotherapist.
e) A radiologist with expertise in pelvic floor imaging.
f) A colorectal surgeon with expertise in pelvic floor problems.
g) A pain specialist with expertise in managing pelvic pain.
The new guidance also suggests mandatory national registry database usage to record all details of surgery for SUI, UUI and POP with annual follow-up to five years post surgery. No surgery for primary SUI, UUI or POP should be offered unless there has been failure of or declination of three-month pelvic floor physical therapy and lifestyle modifications.
First-line surgical options for SUI are:
i) Open or laparoscopic colposuspension
ii) Autologous rectus fascial sling
iii) Retropubic midurethral mesh sling (only with additional detailed guidance as specified in NICE 123).
Intramural bulking agents are to be considered if the above surgical procedures are not suitable or acceptable to the patient.
The transobturator mid-urethral mesh sling may no longer be considered a primary surgical option. This is predicated upon the relative difficulty of removal. It is still a reasonable treatment option in women in whom surgery for SUI is indicated, who are not fit enough for or decline more invasive SUI procedures and in whom it is important to stay out of the pelvis.
The Independent Medicines and Medical Devices Safety (IMMDS) Review
In February 2018 an independent review of the use of surgical mesh, chaired by Baroness Cumberlege, was announced. Baroness Cumberlege made the decision in July 2018 to place an immediate ‘High Vigilance Pause’ (HVP) on use of vaginal mesh for POP and SUI pending the outcome of her review and the updated NICE 2019 guidance on UI and POP [1]. This HVP was extended on 29 March 2019 with an unspecified end date to be when the conditions cited below have been met [11].
Section A:
- Surgeons should only undertake operations for SUI if they are appropriately trained, and only if they undertake operations regularly.
- Surgeons report every procedure to a national database.
- A register of operations is maintained to ensure every procedure is documented and the women identified who have undergone the surgery.
- Reporting of complications via MHRA is linked to the register.
- Identification and accreditation of specialist centres for SUI mesh procedures, for removal procedures and other aspects of care for those adversely affected by surgical mesh.
- NICE guidelines on the use of mesh for SUI are published.
Section B:
- Work with NICE as part of their consultation to strengthen patient information by developing patient decision support tools.
- Specialised commissioning to complete the consultation on the new service specification for complex SUI and POP procedures and mesh removal and procure a small number of designated specialist removal services that will also support urogynaecological / female urology networks.
- Continue to pursue the commissioning of a national clinical audit / registry for urogynaecological procedures for SUI and POP.
Current state of mesh use in UK
Fulfillment of the Mesh Oversight Group Report recommendations
1. Trust appraisal continues in much the same format, however the IMMDS Review has highlighted the importance of this to all medical directors.
2-4. NICE has published its updated guidance [10].
5. A pilot nurse helpline in Scotland received very few calls and was discontinued. It was therefore felt to be an unwarranted use of resource in England.
6. GP e-education packages have been created by the RCGP.
7. Reporting to MHRA has been encouraged by all clinicians, allied health professional and patients with an increase in the number of reports noted – however total number of reports since 2010 remain relatively low.
8. New OPCS codes have been developed [18]:
- M53 Vaginal operations to support outlet of female bladder
- M53.7 Total removal of transobturator tape
- M57 Other vaginal operations to support outlet of female bladder
- M57.1 Introduction of vaginal tape NEC
- M57.2 Total removal of vaginal tape NEC
- M57.3 Partial removal of vaginal tape NEC
- M57.4 Partial removal of transobturator tape
- M57.8 Other specified
- M57.9 Unspecified
9. A one off data gathering exercise on all patient outcomes has NOT occurred. This is, in the authors’ opinion, key to delineating the true extent or not of the problem. A government funded third party review of the outcomes of all women during a set time period having surgical treatment for SUI (mesh and none mesh) is what is actually required to delineate the magnitude of the issue.
10. A registry for all SUI surgery is well on the way to being established by Health Quality Improvement (HQIP).
11-16. A unified PIL for vaginal mesh for SUI has been developed. It is 16 pages long and fairly difficult to read. BAUS have elected to advise members to give this PIL out as mandated along with the new NICE decision aid AND to also provide the BAUS Plain English 10-page PIL and the BAUS options in SUI surgery guide [19-21]. The BAUS PILs are updated every two years.
Fulfillment of the ‘High Vigilance Pause’ conditions
Enhanced Trust Appraisal will fulfill conditions 1 and 2 of Section A of the ‘Pause’. The following have been developed and are at the initial stages of procurement: Specifications for ‘Specialised services for women with complications of mesh inserted for urinary incontinence and vaginal prolapse’ [12], ‘Specialised services for women requiring specialised complex surgery for urinary incontinence and vaginal and uterine prolapse’ [13] and for ‘Specialised complex gynaecology / female urology: genito-urinary tract fistulae (girls and women aged 16 and above)’ [14].
These specifications and NICE 2019 fulfil conditions 1-4 and part fulfil condition 5 in section A and part fulfil condition 2 in section B for cessation of the HVP. The publication of the NICE UI and POP update in April 2019 fulfils condition 6 of section A whilst the publication of the NICE decision aids on surgery for SUI and POP [15-17] fulfils condition 1 of section B.
As per the requirements of the ‘Specialised Services for Mesh Complications’ specification, all women with mesh complications must be discussed at a mesh MDT. Vaginal mesh extrusion into adjacent organs is recognised as an absolute indication for mesh removal. This can be complete or partial – with the best option to be determined by the mesh MDT and the patient. Any surgery will be performed at the mesh MDT centre by the appropriate surgical member(s) of the mesh MDT.
For small vaginal mesh exposures, removal may not always be necessary. If local removal is felt to be a reasonable option by the mesh MDT and the patient, a member (or members) of the regional MDT with the appropriate skill set may perform local vaginal removal. For pain following mesh insertion without exposure, extrusion or erosion, input from a pelvic pain specialist will be necessary. Other associated specialties, such as colorectal, neurology and allied health professionals, will attend and advise the mesh MDT.
As part of the care pathway, all patients with vaginal mesh complications should be referred for initial evaluation to the regional MDT. They should be evaluated in the outpatient setting with a complete history and physical exam along with necessary investigations. Following evaluation their case must be discussed at the mesh MDT and the patient offered complex mesh removal surgery if indicated at the mesh centre, simple mesh removal surgery or observation at the regional MDT centre (if surgery is not indicated, the patient is unfit for surgery or declines surgery) and / or review by the pelvic pain specialist or other appropriate specialists as deemed appropriate by the MDT. At all stages the patient’s view and involvement with their care will be paramount.
Once the appropriate surgical intervention has occurred, all procedures should be recorded on a national database: BAUS, BSUG or the new Registry currently being developed by HQIP. This registry when operational will fulfill condition 3 and 4 of section A and condition 3 of section B of the HVP. Similarly, all adverse events must be reported to the MHRA. It is essential, and part of the specification, that the mesh MDT (along with the regional MDT and the fistula MDT) are supported by MDT administrators and data entry staff to ensure this occurs.
The varying complexity of mesh related complications is matched by the variety of surgeries and techniques available to remove mesh either partially or completely. The Mesh MDT centres must have the capability and competency to offer the full complement of techniques to ensure expeditious treatment for women suffering from mesh related complication. Surgeries to remove mesh include endoscopic management of mesh erosion into the urinary system, vaginal or abdominal removal (both open or laparoscopically), and groin surgery (Figure 2).
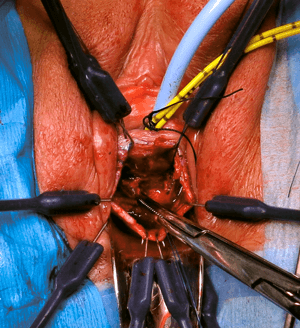
Figure 2: Vaginal excision of mesh extruding into urethra.
The outstanding issues for fulfillment of the HVP conditions are:
- Completion of procurement of the specialist services outlined previously.
- Completion of HQIP registry of procedures with linkage to MHRA.
- Identification and accreditation of specialist centres for SUI mesh procedures – as yet (as far as the authors can determine) no work has been done on this. This is due to the fact that mesh SUI surgery at approximately 7000 cases per year (2017-2018) sits within clinical care group (CCG) commissioning and not specialist commissioning. This issue has been raised at a national level to BAUS Female, Neurological and Urodynamic Urology (FNUU) section, BSUG and NHS England in the last few weeks – and a plan of action is awaited.
Conclusion
No surgeon wishes to do anything other than help their patients and they need evidence in order to determine the best way to do so and avoid harm. It has become clear that whilst mesh surgery for SUI offers a simple, effective treatment for the majority of women, a small but significant number suffer life-changing complications. The scale of this and those at particular risk will remain undetermined until NHS England commissions an independent factual review of the outcomes of all women having mesh and non mesh surgery for SUI. In the meantime whilst the HVP is in place urologists and urogynaecologists should ensure they comply with all the recommendations of the NHS England review in particular MDT discussion, database entry and enhanced consent.
References
1. The Independent Medicines and Medical Devices Safety Review: Written Statement – HCWS841.
www.parliament.uk/business/publications/
written-questions-answers-statements/
written-statement/Commons/2018-07-10/HCWS841/
2. Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 1998;9(4):210-13.
3. Hospital Episodes Statistics.
https://digital.nhs.uk/data-and-information/
publications/statistical/hospital-admitted-patient-care-activity
4. US Food and Drug Administration Center for Devices and Radiological Health. FDA Public Health Notification: Serious Complications Associated with Transvaginal Placement of Surgical Mesh in Repair of Pelvic Organ Prolapse and Stress Urinary Incontinence. 2008.
5. US Food and Drug Administration. Reclassification of Surgical Mesh for Transvaginal Pelvic Organ Prolapse Repair and Surgical Instrumentation for Urogynecologic Surgical Mesh Procedures; Designation of Special Controls for Urogynecologic Surgical Mesh Instrumentation. 2014.
6. Transvaginal mesh implants independent review: final report.
www.gov.scot/publications/scottish-independent
-review-use-safety-efficacy-transvaginal
-mesh-implants-treatment-9781786528711/pages/17/
7. NHS, NICE, BSUG, MHRA, RCOG, BAUS. Mesh Oversight Group Report. 2017.
www.england.nhs.uk/wp-content/
uploads/2017/07/mesh-oversight-group-report.pdf
8. BAUS Patient Information Leaflets.
www.baus.org.uk/patients/information_leaflets/
9. NICE. Transvaginal mesh repair of anterior or posterior vaginal wall prolapse (Interventional procedures guidance [IPG599]). 2017.
www.nice.org.uk/guidance/ipg599
10. NICE. Urinary Incontinence and Pelvic Organ Prolapse in Women: Management (NICE guideline [NG123]). 2019.
www.nice.org.uk/guidance/ng123
11. Extension of Pause to the Use of Vaginal Mesh. 2019.
https://improvement.nhs.uk/documents/5122/
MESH_letter_-_Extension_of_pause_
on_the_use_of_vaginal_mesh_29_March_2019.pdf
12. Service Specifications: Specialised services for women with complications of mesh inserted for urinary incontinence and vaginal prolapse. 2018.
www.engage.england.nhs.uk/consultation/
gynaecology-surgery-and-complex-urogynecology/
user_uploads/complications-of-vagina
l-mesh-draft-service-specification.pdf
13. Service Specifications: Specialised complex surgery for urinary incontinence and vaginal and uterine prolapse. 2018.
www.engage.england.nhs.uk/consultation/
gynaecology-surgery-and-complex-urogynecology/
user_uploads/urinary-incontinence-and
-prolapse-draft-service-specification.pdf
14. Service Specifications: Complex gynaecology/ female urology: genitourinary tract fistulae (girls and women aged 16 and above). 2018.
www.engage.england.nhs.uk/consultation/
gynaecology-surgery-and-complex-urogynecology/
user_uploads/genito-urinary-tract-fistulae
-draft-service-specification.pdf
15. Surgery for stress urinary incontinence. Patient decision aid.
www.nice.org.uk/guidance/ng123/resources/
surgery-for-stress-urinary-incontinence
-patient-decision-aid-pdf-6725286110
16. Surgery for uterine prolapse. Patient decision aid.
www.nice.org.uk/guidance/ng123/
resources/surgery-for-uterine-prolapse
-patient-decision-aid-pdf-6725286112
17. Surgery for vault prolapse. Patient decision aid.
www.nice.org.uk/guidance/ng123/
resources/surgery-for-vaginal-vault
-prolapse-patient-decision-aid-pdf-6725286114
18. OPCS 4 Codes.
https://digital.nhs.uk
19. Synthetic Vaginal Mesh Tape Procedure for the Surgical Treatment of Stress Urinary Incontinence in Women. Patient Information Leaflet. 2017.
www.england.nhs.uk/wp-content/
uploads/2017/11/stress-urinary-incontinence
-mesh-tapes-leaflet-v24.pdf
20. Synthetic Mid Urethral Tapes for Stress Urinary Incontinence (SUI): Information about your procedure from The British Association of Urological Surgeons (BAUS).
www.baus.org.uk/_userfiles/pages/files/
Patients/Leaflets/Synthetic%20sling%20female.pdf
21. Comparison of Treatment Options for Stress Urinary Incontinence (SUI) in Women: Information from the British Association of Urological Surgeons (BAUS).
www.baus.org.uk/_userfiles/pages/files/
Patients/Leaflets/SUI%20options.pdf
Declaration of competing interests: Tamsin Greenwell has obtained education grant funding towards running surgical education courses at UCLH from the following companies: Aspire, Allergan, Astellas, Axonics, Boston Scientific, Contura, Ferring, Laborie, Mediplus, Medtronic, Pierre Fabre, Vesica Urology.






