Priapism is defined as an abnormally persistent erection lasting greater than four hours, not associated with sexual desire [1]. Although relatively uncommon with an incidence of 1.5 per 100,000 [2], priapism has a risk of complications which can have a profound impact on a patient’s quality of life. Consequently, rapid recognition and management is paramount.
There are three distinct types of priapism:
- Ischaemic (also known as low flow)
- Non-ischaemic (also known as high flow)
- Stuttering (also known as recurrent)
Ischaemic priapism is the most common, comprising 95% of all priapism cases [3,4]. Patients usually present with a painful, engorged corpus cavernosum with normal blood flow in the glans and corpus spongiosum [5]. Ischaemic priapism occurs due to failure of the veno-occlusive mechanism resulting in decreased venous outflow. In turn this leads to decreased arterial inflow causing blood stasis, local hypoxia and acidosis [6]. Ischaemic priapism is an emergency due to the risk of erectile dysfunction as a consequence of corporal ischaemia induced fibrosis [7].
Non-ischaemic priapism occurs due to unregulated cavernous arterial inflow and typically presents with a painless, partial erection [5]. This is not an emergency as maintenance of arterial inflow ensures cavernosal tissue oxygenation [6]. Stuttering priapism is characterised by repetitive, painful episodes of prolonged erections [3].
Aetiology
There are multiple causes of failure of the detumescence mechanism:
Haematological [8]
- Sickle cell disease
- Chronic granulocytic leukaemia (50% chance of priapism occurring [9])
- Thromboembolism
Pharmacological [10-20]
- Tamsulosin
- Doxazosin
- Prazosin
- Sildenafil citrate
- Trazodone
- Clozapine
- Chlorpromazine
- Risperidone
- Cocaine
- TPN with 20% fat emulsion
- Intracavernous injections e.g., prostaglandin E, papaverine hydrochloride (Nieminen et al. published a case series in which 21% of priapism cases were caused by intracavernosal injection of a vasoactive drug [21])
Trauma
Blunt perineal or penile trauma is a common cause of non-ischaemic priapism due to fistula formation from the cavernosal artery to corporal tissue [8,10,22]. Trauma can also lead to ischaemic priapism if oedema or a haematoma leads to venous compression.
Neurological
- Spinal cord injury, especially high cord lesions [23]
- Anaesthetic agents
- Lumbar disk disease [24]
Malignant
- Tumours such as bladder, prostate, rectum and their metastasis due to obstruction of venous outflow [25]
Idiopathic
- 30-50% of all priapism events are idiopathic [26].
History and examination
Differentiating between ischaemic and non-ischaemic priapism is necessary in order to assess the level of urgency of intervention. Key points from the history include duration of erection – the British Association of Urological Surgeons (BAUS) guidelines recommend categorising events into <48 hours, 48-72 hours and >72 hours to enable appropriate triaging [27]. Other key points to establish in the history include events surrounding onset of erection, any recent perineal or penile trauma, any previous priapism episodes, past medical history including any underlying haematological disorders, medication history and social history including any illicit drug history. A systems review should be done to identify any red flag symptoms for underlying malignancy and any neurological symptoms.
Examination will allow differentiation between ischaemic and non-ischaemic priapism in most cases. Ischaemic priapism presents as an 100% rigid, painful erection [28]. Non-ischaemic priapism is often described as uncomfortable but not painful and may have associated signs of trauma. Further examination should include an abdominal and rectal exam to look for pelvic malignancy and neurological exam if suspected neurological cause.
During examination in children, applying perineal compression with the thumb may prompt detumescence. This is Piesis sign and signifies non-ischaemic priapism [10].
Investigations and imaging
Blood tests can provide information as to the cause of priapism. BAUS guidelines recommend completing a full blood count, a blood film and an autoimmune screen [27,29]. If there is a suspected haematological cause, haematology should be involved in management.
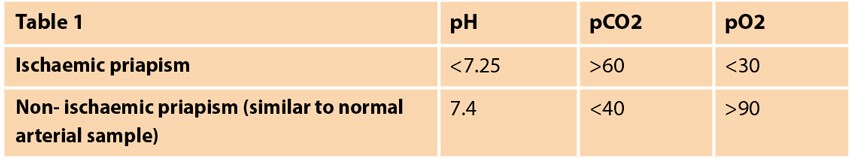
Blood aspiration from the penis should undergo blood gas analysis, allowing identification of ischaemic vs. non-ischaemic priapism as seen in Table 1 [10]. Glucose should also be measured due to research identifying glucopenia as an important prognostic factor [30].
Penile doppler study allows further classification of priapism by allowing identification of cavernous artery inflow and venous sinusoidal outflow velocities [31]. Increased arterial flow in the cavernous arteries is indicative of ischaemic (high flow) priapism [8]. When interpreting this imaging study, it should be remembered that if aspiration or surgery has already been attempted or fibrosis has begun distally, there can be paradoxical raised systolic blood velocity at the base of the shaft of the penis [27]. Penile doppler should be organised urgently however obtaining this imaging should not delay intervention with aspiration as this is both diagnostic and therapeutic.
If there is suspicion of malignancy or in the case of idiopathic priapism, a chest x-ray and CT of the abdomen and pelvis are recommended to look for malignancy.
MRI of the penis can be done to assess hypoxic damage to the corpus cavernosum in cases which are non-responsive to treatment. Additionally, it can be useful when determining suitability for early implantation of penile prosthesis.
In cases of non-ischaemic priapism that are unresponsive to medical treatment, arteriography can be done to locate the best site for embolisation [10].
Management
Treatment should be initiated as soon as possible. The majority of patients presenting with priapism lasting less than 24 hours promptly achieve detumescence however the longer the episode is sustained, the lower the success of treatment.
Ischaemic priapism management
All patients should be given analgesia, prophylactic broad spectrum antibiotics [32] and if necessary, fluids [3].
The first step in management is corporeal aspiration – this has a success rate of around 30% [33]. A local anaesthetic penile block should first be administered then blood aspirated, using a large 19-gauge needle inserted into the corpus cavernosum via the lateral penile shaft or glans penis.
If detumescence does not occur, an alpha-adrenergic agonist such as phenylephrine should be injected at the 3 or 9 o’clock position, avoiding the urethra and neurovascular bundle found on the dorsum of the penis. The recommended dose is 200-250 micrograms, repeated every 10 minutes until detumescence or a maximum dose of 1000 micrograms. During administration it is important to monitor the patient’s observations and to cease treatment with phenylephrine if significant systemic hypertension occurs. Other side-effects include headache, reflex bradycardia, palpitations and arrythmias [34]. If this treatment is given within 12 hours of onset of priapism it has an almost 100% rate of effectiveness [35].
If the use of alpha-adrenergic agonists is contraindicated or the risk of systemic hypertension is too great, there are suggestions in the literature of trialling glycopyrrolate as this has been reported to alleviate intraoperative priapism [36].
The European guidelines for management of priapism include other drugs that can be used as second-line treatment [23].These include:
- Etilephrine
- Adrenaline
- Methylene blue (in priapism due to pharmacological causes [37])
- Terbutaline (used in priapism caused by the intracavernosal injection of vasodilatory drugs. A study by Lowe et al. reported terbutaline as having a response rate of 36% [38])
The BAUS guidelines recommend further management of ischaemic priapism depending upon duration [27].
Under 48 hours
Options include performing a Winter shunt [39] (also known as caverno-glanular shunt) or T shunt under local or general anaesthetic. A Winter Shunt uses a Tru-cut biopsy needle passed through the glans into the corpus cavernosum. A T shunt is performed by inserting an #11 blade through the mid glands into the corpora, rotating it 90 degrees away from the urethra and then withdrawing the blade. Following these procedures, hypoxic blood should be drained from the penis with manual compression until bright red oxygenated blood appears. Once this occurs the wound should be closed with absorbable sutures. If detumescence doesn’t occur, this shunt procedure can be repeated on the contralateral side.
If this shunt procedure fails to resolve the priapism, tunnelling can be considered. Another option to escalate towards is a TTT shunt which involves inserting an 8-F metal dilator through an incision in the glans through to the corpus.
Smooth muscle biopsy should be done intraoperatively and if this reveals smooth muscle necrosis or alternatively if there is failure of shunting, early penile prosthesis implantation should occur within three weeks.
Other less common types of shunt include:
- Al-Ghorab aka caverno-glanular shunt [40] – an incision is made at the corona and the corporal tips are removed allowing blood to flow freely between the glans and corpora.
- Quakles aka caverno-spongiosal shunt [29] – anastomosis between corpus cavernosum and corpus spongiosum. This is done proximally where the corpus spongiosum is thickest as to avoid urethral injury.
The following shunts are technically more difficult to perform and are associated with risk of pulmonary embolism [6,41,42] so should only be done if distal shunting has failed:
- Grayhack aka caverno-saphenous shunt – corpus cavernosum anastomosis with the saphenous vein.
- Caverno-penile dorsal vein shunt – corpus cavernosum anastomosis with the dorsal vein of penis.
It is important that patients are fully informed of the risks and benefits of shunt surgery prior to undergoing the procedure. A key point to ensure patients are aware of is the 50% rate of erectile dysfunction following surgery [46].
48-72 hours
Results of MRI and penile doppler determine management of these cases. If investigations demonstrate perfusion then it is advised to proceed as per previously detailed management for cases under 48 hours. If no perfusion is seen, early penile prosthesis insertion is recommended.
>72 hours
Insertion of early penile prosthesis is recommended by this stage due to it being unlikely that smooth muscle in the corpus cavernosum remains oxygenated and viable [22]. Rees et al. evaluated the satisfaction of patients who had early penile prosthesis insertion due to failure to respond to treatment and found that none of these patients suffered any early complications and that all patients maintained their penile length and were satisfied with the outcome [43]. The biggest risk with penile implantation is infection, consequently it is suggested to give prophylactic antibiotics prior to performing surgery [44]. Due to semi rigid devices being easier to remove in the event of infection, research suggests implanting such devices first line. These can be changed to an inflatable device three to six months down the line if indicated and the patient wishes [45].
Non-ischaemic priapism management
Due to the non-urgent nature and absence of identified risk of penile tissue damage, conservative treatment is the recommended course of action for non-ischaemic priapism [31]. There are reports in the literature of methylene blue in non-ischaemic priapism occurring independent of cavernous artery injury, due to it inhibiting nitrous oxide. However, this isn’t recommended in the guidelines due to associated risk of penile abscess and necrosis [47]. Other side-effects include blue discolouration of the penis and burning sensation [23].
If imaging identifies a fistula as the cause of the non-ischaemic priapism, compression of the fistula should occur. If this fails to initiate detumescence then angiography should be done to allow selective embolisation. This is done with an absorbable material – options include absorbable gelatin or autologous clot [48]. Non-absorbable materials such as metal coils have been used in the past however are rarely used now [10]. It is important to inform patients of the risk of erectile dysfunction occurring post embolisation, however they can be reassured that approximately 80% of patients retain their ability to achieve erections post embolisation [23].
If embolisation fails, surgical ligation of the cavernous artery can be done [10].
Stuttering priapism management
In idiopathic cases in post-pubertal boys only, GnRH agonists and anti-androgens can be trialled in an attempt to prevent future episodes [49]. The European guidelines for treatment of priapism also recognise the reported benefits of using oral alpha-adrenergic agonist pseudoephedrine, digoxin, terbutaline, gabapentin and baclofen [23]. Many of these pharmacological agents take time to work and self-injection of phenylephrine can be useful if episodes of priapism occur in the meantime. This is also recommended in the American guidelines for patients with stuttering priapism who do not respond to treatment [33].
The paradoxical effect of low dose phosphodiesterase-5 inhibitors can also be useful in preventing stuttering priapism however this should not be started during an acute episode [50].
In stuttering priapism cases occurring as a result of sickle cell disease, oral sympathomimetic etilefrine has been reported to have positive results [27] and is consequently recommended on a case by case basis in the European guidelines. There are reports of stilbesterol being used with some success in patients with stuttering priapism as a result of sickle cell disease [51].
Follow-up
As per the BAUS guidelines, all patients presenting with priapism should be followed up long-term in an outpatient clinic [27]. This allows identification of patients with complications arising as a result of priapism such as erectile dysfunction. Medical treatments for erectile dysfunction occurring in this situation include phosphodiesterase-5 inhibitors and intracavernosal prostaglandins. If these prove unsuccessful then penile prosthesis surgery is the final step in treatment.
Complications
It is widely accepted that treatment for ischaemic priapism must occur as soon as possible due to the irreversible damage to the tissue that results from hypoxia [52]. Ultrastructure examination has revealed interstitial oedema in the corpora tissues within 12 hours of onset of ischaemic priapism. By 24 hours there is destruction of the sinusoidal endothelium and exposure of the basement membrane to which platelets adhere. At 48 hours vascular thrombi are seen to occur which are responsible for ischaemic and necrosis [3] that leads to local inflammation and fibroblast proliferation [29]. It is this mechanism which results in the inability of the sinusoids in corpora cavernous to distend to create an erection, leading to erectile dysfunction as a complication of ischaemic priapism [6,7]. Ninety percent of patients with priapism lasting beyond 24 hours develop erectile dysfunction according to research by Pryor et al. [53].
Other complications associated with priapism include6:
- Recurrence
- Bleeding
- Infection
- Skin necrosis [10]
- Urethro-cutaneous fistula
- Urethral stricture
Sickle cell disease
Priapism is seen in 38-42% of men with sickle cell anaemia and as many as 63% of priapism cases in children are in those with sickle cell disease [8]. There is a bimodal peak of incidence occurring at 5-13 years and 21-20 years [7]. It is more likely to develop in individuals with lower Hb F levels, lower reticulocyte levels, increased platelet counts and the Hb SS genotype. It usually presents as an ischaemic, bicorporal priapism which has begun as a physiological erection. Stasis of blood in the corpora occurs during the erection and in patients with sickle cell, the resultant hypoxia and acidosis leads to sickling of red blood cells and venous outflow obstruction [7,54]. Priapism in men with sickle cell disease can also present as stuttering, usually occurring at night.
Management for acute ischaemic priapism in patients with sickle cell involves conservative treatment with intravenous fluids, oxygenation and systemic alkalinisation to prevent further sickling. Aspiration and irrigation with saline and phenylephrine should then be tried and if this fails, partial exchange transfusion with the aim to reduce HbS <30% with total Hb 10g/dL. However, this should not be done first line due to its potential neurological side-effects [55].
Options for treatment to prevent repeated episodes of priapism in sickle cell patients including etilefrine (first line), stilbesterol, bicalutamide or pseudoepinephrine. Shunt surgery is reserved for treatment resistant cases [7].
It is important to involve haematologists in the management of these patients as individuals with sickle cell that have priapism episodes have been found to have higher incidence of stroke, pulmonary hypertension, renal failure, leg ulcers and premature death [54].
Conclusion
Although relatively uncommon, medical education on priapism is important to enable prompt identification and treatment. Priapism should be assessed on a case by case basis as to the most appropriate initial treatment, in order to maximise likelihood of timely detumescence and consequently minimise the occurrence of impotence and other serious complications.
References
1. Smith A, Axilrod A. Penn Clinical Manual of Urology Saunders/Elsevier; 2007.
2. Eland I, van de Lei J, Stricker B, Sturkenboom M. Incidence of priapism in the general population. Urology 2001;57(5):970-9.
3. Cheng L, MacLennan G, Bostwick D. Penis and Scrotum, Urologic Surgical Pathology (4th ed): Elsevier; 2020.
4. Broderick GA, Kadioglu A, Bivalacqua TJ, et al. Priapism: pathogenesis, epidemiology, and management. J Sex Med 2010;7(1 Pt 2):476-500.
5. Latinin J. Clinical Men’s Health, Evidence in Practice Elsevier; 2008.
6. El-Bahnasawy M, Dawood A, Farouk A. Low‐flow priapism: risk factors for erectile dysfunction. BJU International 2002;89(3):285-90.
7. Saunthararajah Y, Vichinsky EP. Hematology (7th Edition) Elsevier; 2018.
8. Melman A, Serels S. Priapism. International Journal of Impotence Research 2000;12(Suppl 4):S133-9.
9. Schreibman SM, Gee TS, Grabstald H. Management of priapism in patients with chronic granulocytic leukemia. J Urol 1974;111:786-8.
10. Cherian J, Rao AR, Thwaini A, et al. Medical and surgical management of priapism. Postgrad Med J 2006;82:89-94.
11. Dodds PR, Batter SJ, Serels SR. Priapism following ingestion of tamsulosin. J Urol 2003;169:2302.
12. Avisrror MU, Fernandez IA, Sanchez AS, et al. Doxazosin and priapism. J Urol 2000;163:238.
13. Bhalla AK, Hoffrand BI, Phatak PS, et al. Prazosin and priapism. Br Med J 1979;2:1039.
14. Sur RL, Kane CJ. Sildenafil citrate-associated priapism. Urology 2000;55:950.
15. Saenz de Tejada I, Ware JC, et al. Pathophysiology of prolonged penile erection associated with trazodone use. J Urol 1991;145:160-4.
16. Compton MT, Miller AH. (2001). Priapism associated with conventional and atypical antipsychotic medications: A review. The Journal of Clinical Psychiatry 2001;62(5):362-6.
17. Altman AL, Seftel AD, Brown SL, Hampel N. Cocaine associated priapism. J Urol 1999;161:1817-18.
18. Aronson J. Meyler’s Side Effects of Drugs (16th ed). Elsevier; 2016.
19. Ekström B, Olsson AM. Priapism in patients treated with parenteral nutrition. Br J Urol 1987;59:170-1.
20. Porst H. The rationale for prostaglandin E1 in erectile failure. A survey of world‐wide experience. J Urol 1996;155:802.
21. Nieminen P, Tammala T. Aetiology of priapism in 207 patients. Eur Urol 1995;28:241-5.
22. Salonia A, Eardley I, Giuliano F, et al. Guidelines on Priapism. European Association of Urology; 2014.
23. Bedbrook G. ‘Medical management’ In: The Care and Management of Spinal Cord Injuries Spinger-Verlag; 1981.
24. Baba H, Maezawa Y, Furusawa N, et al. Lumbar spinal stenosis causing intermittent priapism. Paraplegia 1995;33:338–45.
25. Schroeder‐Printzen I, Vosshenrich R, Weidner W, Ringart RH. Malignant priapism in a patient with metastatic prostate adenocarcinoma. Urol Int 1994;52:52-4.
26. Benson GS. Priapism. AUA Update Series 1996;XV (Lesson 11):86-91.
27. Muneer A, Brown G, Dorkin T, et al. BAUS consensus document for the management of male genital emergencies: priapism. BJU International 2018;121(6):835-9.
28. Brock G, Breza J, Lue TF, Tanagho EA. High Flow priapism: a spectrum of disease. J Urol 1993;150:968-71.
29. Keoghane S, Sullivan M, Miller M. The aetiology, pathogenesis and management of priapism. BJU International 2002;90(2):149-54.
30. Muneer A, Cellek S, Dogan A, et al. Investigation of cavernosal smooth muscle dysfunction in low flow priapism using an in-vitro model. Int J Impot Res 2005;17:10-18.
31. Hatzichristou D, Salpiggidis G, Hatzimouratidis K, et al. Management strategy for arterial priapism: therapeutic dilemmas. Journal of Urology 2002;168(5):2074-7.
32. Kulmala R. Treatment of priapism: primary results and complications in 207 patients. Ann Chir Gynaecol 1994;83:309-14.
33. Montague DK, Jarow J, Broderick GA, et al. American Urological Association guideline on the management of priapism. J Urol 2003;170:1318-25.
34. Montague D, Jarow J, Broderick G, et al. Management of Priapism. American Urological Association; 2010.
35. Kulmala RV, Tamella TL. Effects of priapism lasting 24 hours or longer caused by intracavernosal injection of vasoactive drugs. Int J Impot Res 1995;7:131-6.
36. Valley MA, Sang CN. Use of glycopyrrolate to treat intraoperative penile erection. Case report and review of the literature. Reg Anesth 1994;19:423-8.
37. Martinez Portillo FJ, Hoang‐Boehm J, Weiss J, et al. Methylene blue as a successful treatment alternative for pharmacologically induced priapism. Eur Urol 2001;39:20-3.
38. Lowe FC, Jarrow JP. Placebo controlled study of oral terbutaline and pseudoephedrine in the management of prostaglandin induced prolonged erections. Urology 1993;42:51-3.
39. Winter CC. Priapism cured by creation of a fistula between the glans penis and the corpora cavernosa. J Urol 1978;119:227-8.
40. Ercole CJJ, Pontes JE, Pierce JM Jr. Changing surgical concepts in the treatment of priapism. J Urol 1981;125:210-11.
41. Grayhack JT, McCullough W, O’Connor VJ Jr. Venous bypass to control priapism. Invest Urol 1964;1:509-13.
42. Barry JM. Priapism: treatment with corpus cavernosum to dorsal vein of the penis shunt. J Urol 1976;116:754-6.
43. Rees RW, Kalsi J, Minhas S, et al. The management of low-flow priapism with the immediate insertion of a penile prosthesis. BJU Int 2002;90:893-7.
44. Zacharakis E, Garaffa G, Raheem A, et al. Penile prosthesis insertion in patients with refractory ischaemic priapism: early vs delayed implantation. BJU International 2014;114(4):576-81.
45. Ralph DJ, Garaffa G, Muneer A, et al. The immediate insertion of a penile prosthesis for acute priapism. Eur Urol 2009;56:1033-8.
46. Bertram RA, Webster GD, Carson CC III. Priapism: etiology, treatment and results in series of 35 presentations. Urology 1985;26:229-32.
47. Perry PM, Meinhard E. Necrotic subcutaneous abscess following injection of methylene blue. Brit J Clin Pract 1974;28:289.
48. Walker TG, Grant PW, Goldstein I, et al. High flow priapism: treatment with superselective transcatheter embolization. Radiology 1990;174:1053-4.
49. Costabille RA. Successful treatment of stutter priapism with an antiandrogen. Techn Urol 1998;4:167-8.
50. Burnett A, Bivalacqua T, Champion H, Musicki B. Long-term oral phosphodiesterase 5 inhibitor therapy alleviates recurrent priapism. Urology 2006;67(5):1043-8.
51. Serjeant GR, de Ceuler K, Maude GH. Stilbestrol and stuttering priapism in homozygous sickle-cell disease. Lancet 1985;2:1274-6.
52. Spycher MA, Hauri D. The ultrastructure of the erectile tissue in priapism. J Urol 1986;135:142-7.
53. Pryor J, Akkus E, Alter G, et al. Priapism. The Journal of Sexual Medicine 2004;1(1):116-20.
54. Steinburg M. Goldman’s Cecil Medicine, Sickle cell Disease and other haemoglobinopathies (24th ed). Elsevier; 2012.
55. Siegel JF, Rich MA, Brock WA. Association of sickle cell disease, priapism, exchange transfusion and neurological events: ASPEN syndrome. J Urol 1993;150:1480-2.
Declaration of competing interests: None declared.






