Background
Surgery for pelvic organ prolapse (POP) is common among women. The lifetime risk of undergoing at least one surgical procedure for POP is up to 20% [1]. This kind of surgery will be increasingly important due to an ageing population and, given the significant resources required in the future, it will be crucial to perform effective, durable and cost-effective interventions with minimal morbidity.

Following a long tradition, prolapse surgery is efficiently performed with native tissue repair techniques although there is a lack of consensus about the optimal surgical approach and several publications reporting failure rates of up to 30% [2]. Although these exaggerated numbers are more the result of the heterogeneity in surgical skill levels than imperfections of the techniques themselves, rapid industrial development, paired with amplified marketing strategies, led to the search for alternative approaches. Subsequently, out of the successful use of synthetic mesh in the surgical treatment of abdominal hernia emerged the belief that a similar approach could be of some benefit in pelvic surgery.
Historically, the use of mesh in gynaecology began in the 1970s, with open abdominal POP repair which was rapidly transferred to laparoscopy with a trend to offer it to younger patients. Transvaginal mesh (TVM) use for POP surgeries became FDA-cleared in 2004 and gained in popularity with a trend boosted by encouraging results after mid-urethral sling placement for stress urinary incontinence in the late 1990s. In 2006, at the peak of synthetic mesh use for POP, one-third of all prolapse operations involved some mesh use; we were at the peak of the so called ‘Hype Cycle’, a theoretical concept developed and branded by the Gartner company, an information technology advisory and research firm. It illustrates how a technology will evolve over time (Figure 1).
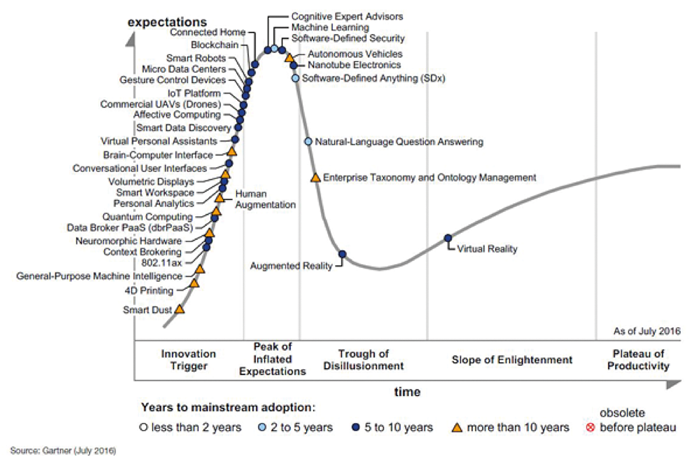
Figure 1: The Hype Cycle for Emerging Technologies, 2016
(http://www.gartner.com/newsroom/id/3412017, accessed 8 August 2017).
Since then, there has been intense debate about the use of synthetic meshes in POP surgery, given the existence of a highly efficient alternative, which is traditional native tissue repair. Although a graft inlay seems to reduce the risk of recurrence, a main complication related to its use is erosion in the vagina which is particularly an issue when it is placed vaginally. In 2011, after the FDA issued a warning concerning transvaginal mesh kits, many of these kits were voluntarily withdrawn from the market under economic and juridical pressure and the debates were increasingly dominated by emotion rather than scientific facts. Although there is a decrease in the use of meshes, there has been significant improvement in the quality of material with promising results in the hands of skilled surgeons familiar with traditional techniques.

“In 2006, at the peak of synthetic mesh use for POP, one-third of all prolapse operations involved some mesh use.”
Given the numerous guidelines and recommendations issued by scientific societies and national healthcare regulators over the past years, our aim is to provide an answer to the question “where do we stand today with the use of meshes in POP surgery?”
Anatomic considerations
The principle of POP surgery is a defect-orientated repair based on anatomic considerations as suggested by De Lancey et al. [3]. Level 1 defects refer to the mid-vagina at the level of the uterosacral ligaments resulting in an enterocele, a uterine prolapse or prolapse of the vault after hysterectomy. A Level 2 defect refers to the anterior and posterior lower vagina leading to a cystocele and / or a rectocele. Level 3 defects are located sub-urethrally causing stress urinary incontinence for which gold standard treatment would be the placement of a mid-urethral sling mesh.
Why use mesh?
The rationale behind the use of mesh for POP surgery was the thought of potential reduction of the high recurrence rates after native tissue repair (vaginal hysterectomy, anterior and posterior repair and apex fixating techniques such as sacrospinous ligament suspension, uterosacral ligament suspension or McCall’s culdoplasty). Meshes intend to reinforce muscles and ligaments of the pelvic floor in order to restore the functional anatomy. They must be biologically safe, chemically and physically inert, non-carcinogenic and mechanically solid while allowing extension flexibility. As sterile devices, they should not initiate any allergic or inflammatory response by the host. Currently, none of the marketed meshes fulfils these criteria.
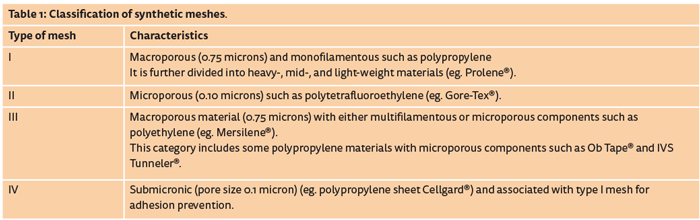
Four varieties of meshes have been developed for use in pelvic floor surgery: autografts from fascia lata or the rectus sheath, allografts from human cadavers, xenografts from bovine or porcine material, and synthetic grafts. They can be manufactured as absorbable or non-absorbable grafts. Commonly used synthetic materials are different varieties of polyester and polypropylene. They are classified depending on various factors influencing their interaction with the surrounding tissue: pore size, weight and structure (mono or multifilament) [4] (Table 1).
Which mesh and how? Abdominal or vaginal?
The assessment of mesh augmented surgery differs widely whether the graft is placed abdominally or vaginally. There is robust evidence that the anatomical outcome for polypropylene mesh is better as compared to biological graft [1].
After the initial success of transvaginal mesh kits, numerous companies bypassed clinical trials, US law requiring manufacturers only to show that their product is substantially equivalent to one already on the market. As a consequence, more than 40 companies began the manufacturing of mesh devices in the 10 years following the initial clearance in 2004 and numerous products and surgical kits were marketed before evidence of its benefit was established.
The most common complications were vaginal mesh exposure (up to 26%), infection, pain, dyspareunia (17-63%), bleeding, urinary symptoms and even organ perforation with many of these complications requiring surgical correction [5,6]. As a consequence, numerous manufacturers, after being confronted with costly law suits, withdrew their devices from the market. In January 2016, the FDA reclassified transvaginal mesh devices from moderate risk to high risk (class III), after indicating in May 2014 that such action was necessary.
In the future, this reclassification requires a premarket approval application to be led for each device, with safety and efficacy demonstrated. Despite strengthened regulations, newer light-weight transvaginal polypropylene mesh products have been introduced after well conducted scientific studies reported subjective success rates of more than 90% at one year, a low reoperation rate for mesh exposure of 1.3% and a satisfaction rate of 98% [7]. Many national guideline committees as well as major scientific societies recommend the exclusive use of Type I meshes both in vaginal and abdominal surgery.
Abdominal POP repair using mesh is associated with significantly lower complication rates than the vaginal approach. Sacrocolpopexy or hysteropexy are both well-established techniques and can be performed with a laparotomy, laparoscopically or with robotic assistance. The laparoscopic approach is associated with lower blood loss, longer operating time and shorter hospital stay than the abdominal approach and there is no strong evidence about any benefit when using the robot. The laparoscopic approach is now considered a gold-standard for apical repair (Level 1) [5]. Lateral suspension has been proposed as an efficient alternative of the laparoscopic route, avoiding morbidity due to sacral dissection [8].
What’s the evidence?
Level 1 repair
Apical compartment prolapse occurs either with the uterus, the cervix after supracervical hysterectomy or with the vaginal vault after total hysterectomy. Laparoscopic sacrocolpopexy (vault or cervix) and sacral hysteropexy (uterus present) with synthetic mesh are both minimally invasive procedures with high anatomic (objective) success rates of over 90%, which is superior to the results with native tissue repair [5]. The role of the uterus in apical compartment prolapse repair is highly debated.
Traditionally, hysterectomy was regularly performed since the uterus was considered as playing an active role in POP occurrence. There is now evidence of a more passive role of the uterus and it is reasonable to consider uterus-preserving surgery in the absence of a formal indication for hysterectomy. However, the reoperation rate for prolapse seems to be higher for hysteropexy whereas the mesh exposure rate is higher after hysterectomy. The benefit of sacral colpopexy performed after concomitant supracervical hysterectomy has to be counterweighed against the risk associated with morcellation and unanticipated pathology such as endometrial cancer (0.3%) or sarcoma [1]. Numerous studies have provided evidence that sacropexy with polypropylene mesh has higher success rates not only when compared to native tissue repair techniques but also with transvaginal mesh techniques [5,9].
There is a lower risk of awareness of prolapse, recurrence and re-operation whereas laparoscopy is associated with longer operating time and higher costs. Current evidence does not suggest any benefit of vaginal mesh surgery over native tissue repair in terms of objective and subjective outcome, surgery for recurrence or occurrence of dyspareunia. Traditional vaginal techniques remain highly efficient alternatives which can be performed in loco-regional anaesthesia in patients with significant comorbidities.
Level 2 repair
Although laparoscopic sacropexy with mesh does primarily intend to treat apical prolapse, numerous surgeons perform deep dissection of the vesico-vaginal space and / or the recto-vaginal space, concomitantly correcting a cystocele or a rectocele respectively [6,8]. For the vaginal approach of the anterior compartment, there is robust evidence that the anatomic outcome with mesh augmentation is better when compared to anterior colporrhaphy. Subjective outcomes were also reported as improved although they were not evaluated with validated questionnaires. However, vaginal mesh surgery is associated with longer operating time, greater blood loss, higher rate of bladder injury and, above all, mesh extrusion occurs in 11.5%. For the vaginal posterior compartment repair, there is no evidence for a benefit when using a mesh compared to posterior colporrhaphy [1,10].
Conclusion
The use of synthetic mesh in laparoscopic prolapse surgery is safe and efficient both in a uterus-conserving setting and when associated with hysterectomy. For the vaginal route, current evidence about the risk-benefit profile of transvaginal mesh does not support routine use in primary surgery given similar outcomes associated with higher complication rates, although a significant number of the studied material is no longer available. Transvaginal mesh surgery remains an alternative for recurrent prolapse repair and there has been significant improvement in the quality of mesh material with promising results in large series. These results, however, need to be confirmed in further clinical trials. Referring to Gartner’s Hype Cycle, we are probably currently climbing the slope of enlightenment after having crossed the valley of disillusionment and are heading towards a plateau of productivity. At the end, various factors such as patient’s age, fertility preservation and level of defect should be taken into account when it comes to surgical planning. Above all, in the context of functional surgery, a woman’s individual goals, as well as her individual risks of surgical complications, recurrence and de novo symptoms should determine the choice of procedure, where the use of new synthetic mesh material remains an option in the hands of skilled laparoscopic and vaginal surgeons.
We invite you to join us at ICS 2018 in Philadelphia, where this topic will be discussed further.
References
1. Maher C, Baessler K, Barber M, et al. Pelvic Organ Prolapse Surgery. In: Abrams P, Cardozo L, Wagg A, Wein A (Eds.). Incontinence 6th Edition International Continence Society; 2017.
2. Paz-Levy D, Yohay D, Neymeyer J, et al. Native tissue repair for central compartment prolapse: a narrative review. Int Urogynecol J 2017;28(2):181-9.
3. DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 1992;166(6 Pt 1):1717-24; discussion 24-8.
4. Amid PK, Shulman AG, Lichtenstein IL, et al. Biomaterials for abdominal wall hernia surgery and principles of their applications. Langenbecks Arch Chir 1994;379(3):168-71.
5. Maher C, Feiner B, Baessler K, et al. Surgery for women with apical vaginal prolapse. Cochrane Database Syst Rev 2016;10:CD012376.
6. Maher C, Feiner B, Baessler K, et al. Surgery for women with anterior compartment prolapse. Cochrane Database Syst Rev. 2016;11:CD004014.
7. Altman D, Mikkola TS, Bek KM, et al. Pelvic organ prolapse repair using the Uphold Vaginal Support System: a 1-year multicenter study. Int Urogynecol J 2016;27(9):1337-45.
8. Veit-Rubin N, Dubuisson JB, Gayet-Ageron A, et al. Patient satisfaction after laparoscopic lateral suspension with mesh for pelvic organ prolapse: outcome report of a continuous series of 417 patients. Int Urogynecol J 2017 [Epub ahead of print].
9. Maher C, Feiner B, Baessler K, et al. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database Syst Rev 2016;2:CD012079.
10. Glazener CM, Breeman S, Elders A, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel-group, multicentre, randomised, controlled trials (PROSPECT). Lancet 2017;389(10067):381-92.
Declaration of competing interests: None declared.

*International Continence Society
www.ics.org