Introduction
Retroperitoneal fibrosis (RPF) is a condition that occurs when extensive fibrosis develops in the retroperitoneum, usually centred over the anterior aspect of the fourth and fifth lumbar vertebrae. The fibrotic tissue typically surrounds the infrarenal aorta, inferior vena cava and iliac vessels.
RPF is idiopathic in more than two-thirds of cases, the remainder being due to other causes, including malignancy, drugs and infections. Idiopathic RPF has a good prognosis, compared with malignant RPF where average survival is reported to be three to six months [1].
Making the diagnosis of RPF can be challenging as the symptoms are often vague and there are no specific tests for RPF. Blood tests are non-specific, but include elevated erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and alkaline phosphatase [2]. If the diagnosis is made promptly and treatment begins in a timely manner, benign RPF has a good prognosis. The fibrosis can lead to ureteric obstruction and hydronephrosis.
Imaging plays a vital part in both the diagnosis and exclusion or confirmation of complications related to the RPF. Imaging features of the fibrosis and common complications will be described in this article.
Clinical presentation
Symptoms are often non-specific and may include a dull pain which can be in the back, flank or lower abdomen [3]. Patients may also present with high temperatures, lower limb oedema, weight loss and anorexia, or symptoms due to ureteric obstruction. It is twice as common in males as females and has a peak incidence in adults aged 40-60 years.
“Treatment is given in order to relieve the ureteric obstruction, decrease the risk of systemic disease and to prevent disease progression or recurrence.”
There is often an association with an atherosclerotic aorta but the exact reason for the fibrosis to develop is often unclear. The fibrosis leads to medial deviation of the ureters, in comparison with other retroperitoneal processes, such as lymphoma, where the ureters will be deviated medially. This is an imaging finding that is vital in the diagnosis of RPF.
Biopsy will often just show fibrotic or inflammatory tissue rather than lymphomatous type cells, but it can sometimes be difficult to obtain accurate tissue diagnosis due to the location of the fibrosis and the fact that when biopsies are performed, only fragments of tissue may be acquired.
Imaging
Plain radiographs are of limited use in the diagnosis. If stents are in place, or excretory phase imaging is performed, medial deviation of the ureters may be seen (Figure 1). Medial deviation of the ureters occurs due to the fibrotic tissue pulling the ureters medially, usually in the middle third at the level of L3/L4. Medial deviation of the ureters is seen in approximately 80% of patients with RPF, which is the most common cause.
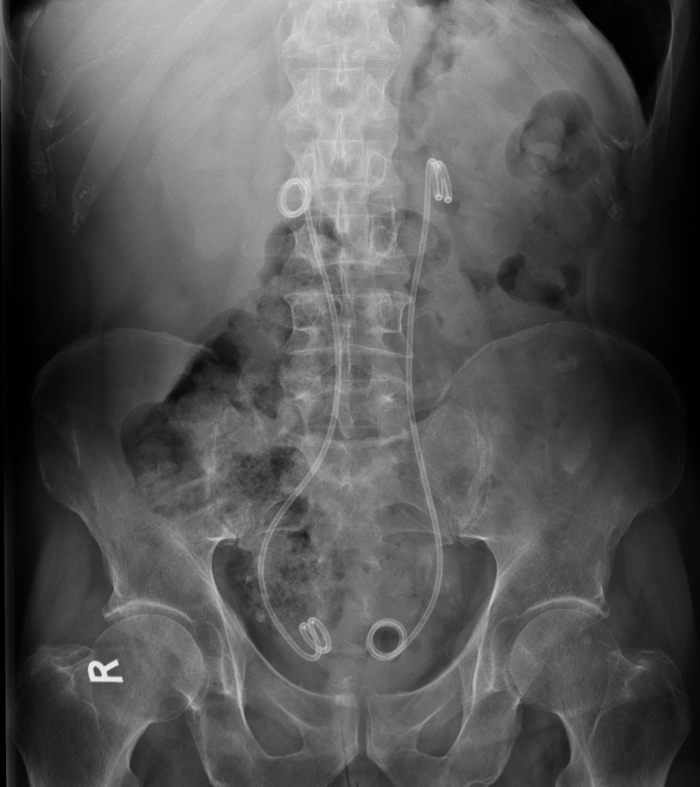
Figure 1: AXR of a patient with bilateral ureteric stents demonstrates medial deviation of both ureters which overlie the vertebral bodies rather than transverse processes, most marked in the mid-ureters.
CT is the primary imaging modality used in patients with RPF. It allows evaluation of the location and extent of the fibrosis, as well as assessment for complications including ureteric obstruction or invasion into other vascular structures. CT usually shows an irregular soft tissue mass surrounding the aorta and enveloping the ureters and IVC (Figure 2). The soft tissue has similar Hounsfield units to muscle and the fat plane between the tissue and the psoas may be obliterated.
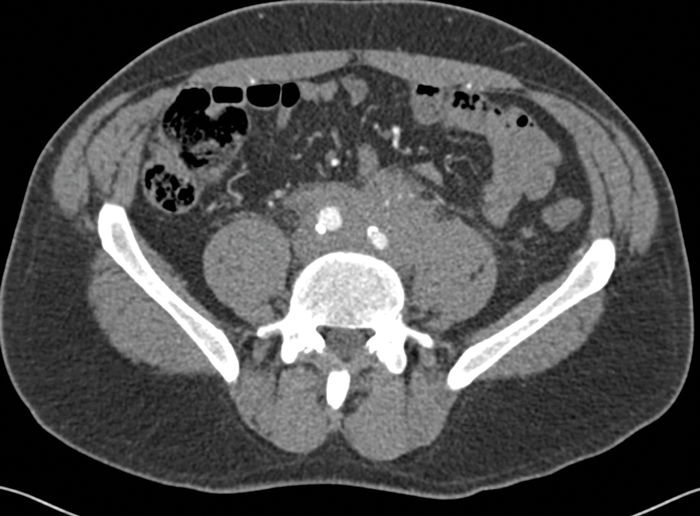
Figure 2: Arterial phase CT abdomen shows a rim of soft
tissue surrounding the anterior aspect of the aorta.
The ureters may be medially deviated due to the fibrotic tissue, rather than laterally deviated as is seen in cases of lymphoma or extensive retroperitoneal lymphadenopathy from other causes. The fibrotic tissue may be seen to extend to encase the common iliac arteries as well as the aorta, depending on the extent of the disease (Figure 3).
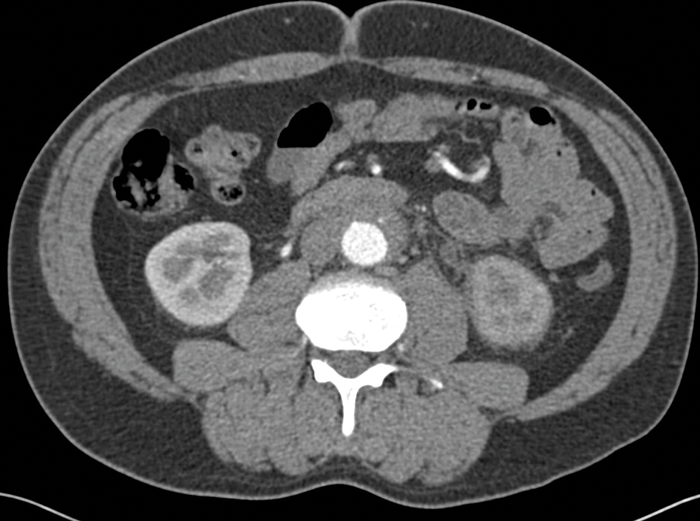
Figure 3: Arterial phase CT abdomen shows the soft tissue extending inferiorly to encase the iliac arteries.
There is loss of the fat plane between the fibrotic tissue and the left psoas muscle.
CT also allows detection of other causes of RPF, including malignancy, and is useful for follow-up purposes to assess if the fibrotic tissue is responding to treatment (Figure 4).
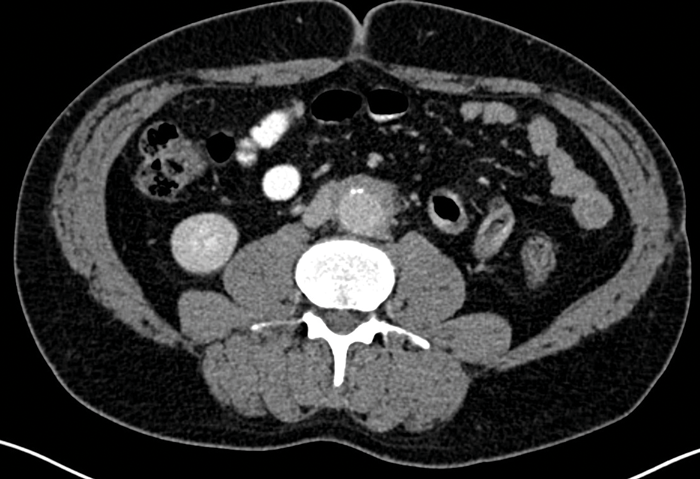
Figure 4: Portal venous phase CT abdomen in the same patient as Figures 2 and 3 showing that the
fibrotic tissue has markedly decreased in size compared with previous imaging, as a result of successful treatment.
Ideally contrast is used, but if patients already have renal impairment due to ureteric obstruction, then non-contrast CT abdomen can be performed.
MRI can be used, particularly in cases in young patients where follow-up imaging is being performed. This decreases the radiation dose to the patient whilst enabling the fibrotic tissue and complications to be assessed. The fibrotic tissue is usually of low signal on T1 imaging and of variable signal on T2 imaging. Differentiation between benign and malignant RPF is difficult with MRI. Dynamic contrast enhanced MRI can be used for follow-up purposes, to monitor response to treatment [4].
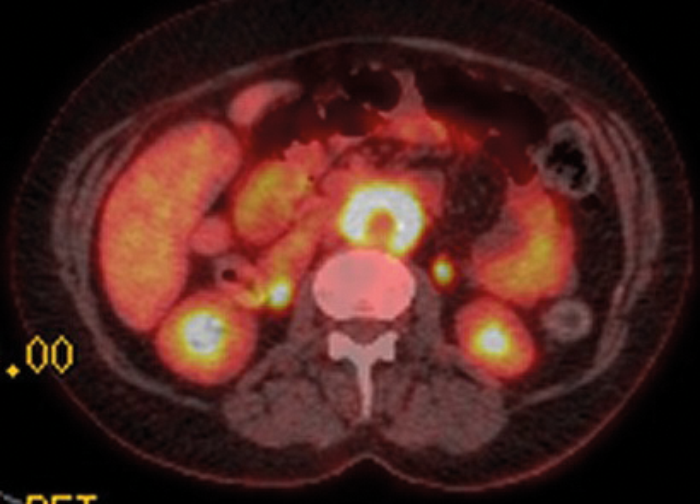
Figure 5: Axial fused PET-CT shows extensive uptake and metabolic activity encasing the aorta.
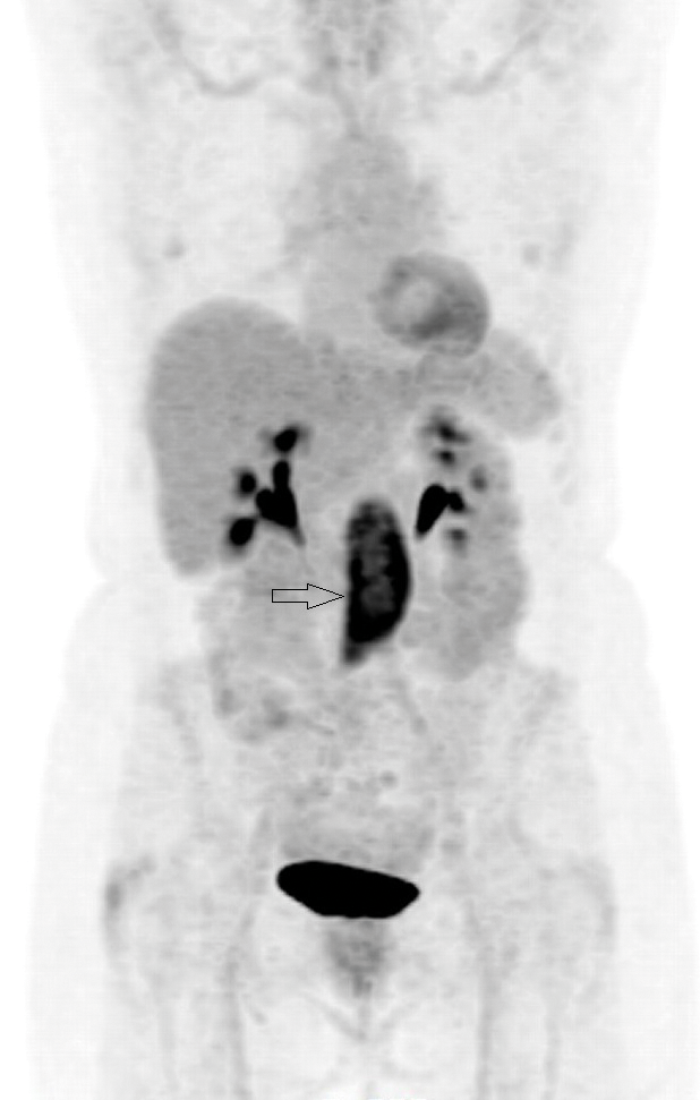
Figure 6: Coronal PET-CT image shows intense uptake
in the tissue seen on the axial image (arrow).
PET-CT with 18F-fluorodeoxyglucose (18F-FDG) can be used to ascertain if there is functional tissue with areas of increased glucose activity. Active RPF is seen as an area of increased uptake (Figures 5 and 6) and this can be used to assess if treatment has been successful. Uptake is seen in active inflammation but not if there is metabolically inactive disease [5]. However, lymphoma also demonstrates avid 18F-FDG uptake, so PET-CT cannot give a definitive diagnosis, but needs to be interpreted in conjunction with other imaging modalities.
Other differential diagnoses include metastatic nodal disease or lymphoma. Lymphoma should be considered if the rim of tissue seen in the retroperitoneum is centred superior to L4/5 level. If the diagnosis is still unclear and lymphoma is clinically suspected, biopsy of the retroperitoneal tissue may be performed. This is usually done under radiological guidance, but can be performed laparoscopically if the tissue is not easily targeted percutaneously.
Ultrasound is of little value, apart from identifying that there is hydronephrosis and renal obstruction. Soft tissue may be seen adjacent to the aorta, but this is relatively poorly visualised and not reliable in either making the diagnosis or follow-up.
Treatment
There is currently little evidence regarding the best management of these patients as no controlled drug-related trials have been undertaken.
Treatment is given in order to relieve the ureteric obstruction, decrease the risk of systemic disease and to prevent disease progression or recurrence. Corticosteroids are usually the first-line medical treatment given and in most patients, leads to improvement of symptoms. A decrease in the size of the retroperitoneal mass is often seen, and ureteric obstruction will be seen to slowly resolve.
Before treatment commences, if there is ureteric obstruction, ureteric stenting is often required. The medial deviation of the ureters can be seen on abdominal X-Ray if stents are in place, as the stent can be visualised in the lines of the ureters (Figure 1).
Surgical treatment such as ureterolysis can be performed to attempt to alleviate the obstruction and biopsy can be obtained at the same time if required. The main purpose of surgical treatment is to relieve the ureteric obstruction rather than attempt to remove the fibrotic tissue completely.
Conclusion
CT is the first-line imaging modality for assessment of patients with RPF and can also be used for follow-up purposes. However, in young patients, MRI should be considered for follow-up imaging as it does not involve ionising radiation.
References
1. Arrive L, Hricak H, et al. Malignant versus non-malignant retroperitoneal fibrosis: differentiation with MR imaging. Radiology 1989;172(1):139-143.
2. Barrison IG, Walker JG, et al. Idiopathic retroperitoneal fibrosis – is serum alkaline phosphatase a marker of disease activity? Postgrad Med J 1988;64(749):239-41.
3. Yachoui R, Sehgal R, Carmichael B. Idiopathic retroperitoneal fibrosis: clinicopathological features and outcome analysis. Rheumatol Clin 2016;35(2):401-7.
4. Burn PR, Singh S, et al. Role of gadolinium-enhanced magnetic resonance imaging in retroperitoneal fibrosis. Can Asso Radiol J 2002:53(3):168-170.
5. Caiafa RO, Vinuesa AS, Izquierdo RS et al. Retroperitoneal fibrosis: role of imaging in diagnosis and follow-up. Radiographics 2013;33(2):535-52.




