Prior to 1980, surgeons had been struggling to provide a catheterisable, continent channel as an alternative to the native urethra, primarily for paediatric patients with congenital neuropathic bladder. In 1980, Professor Paul Mitrofanoff described the continent supravesical antireflux appendicovesicostomy [1] in which the appendix is harvested on its vascular pedicle and refashioned into a catheterisable continent channel passing in an antireflux manner into the native bladder or neobladder. This procedure provides a short, straight and easily catheterisable channel that has shown longevity and a relative lack of surgical and metabolic complications.
Since then the Mitrofanoff principle has been expanded utilising many other tissues, most commonly reconfigured small bowel (Yang [2], Monti et al. [3]) but also utilising colon, ureter, stomach, fallopian tube and vas deferens [4,5]. In 1990 Malone et al. combined the Mitrofanoff principle with the concept of an antegrade continence enema (MACE) to create the MACE for treatment of faecal incontinence and / or constipation [6].
The appendix is the conduit of first choice because of ease of use and relative lack of complications and should be used in preference for MACE formation if synchronous MACE and Mitrofanoff / Monti channel is planned [7]. Since 1997 in situ appendix has been the technique of choice for MACE formation, although the use of MACE has significantly reduced of late.
“The patient must have sufficient manual and mental dexterity to perform stomal catheterisation and understand the need for life-long follow-up, and the pros and cons of the procedure and the alternatives.”
In this article we will discuss the indications, preoperative counselling, operative technique, alternatives and surgical variations of the Mitrofanoff channel as well as its long-term management and outcomes.
Indications
The Mitrofanoff channel was originally described as a technique for the restoration of continent bladder emptying in children with congenital neuropathic bladder dysfunction such as spinal dysraphism [1]. Since its inception it has developed a range of applications in this form, in both adult and paediatric populations. These include replacing a damaged, absent or structurally abnormal urethra in the following situations [1,8-10]:
- Traumatic loss (pelvic fracture or gunshot) with failed reconstruction
- Congenitally absent urethra
- Urethrectomy for urethral malignancy
- Prune belly syndrome
- Cloacal exstrophy
- Sacral agenesis
- Bladder exstrophy
- Episapdias
- Posterior urethral valves
- Anorectal agenesis
- Multiple sclerosis
- Spinal cord injury
Alternatively a Mitrofanoff channel can be tunnelled into a bowel neobladder to form a continent urinary diversion following cystectomy and reconstruction for a range of indications including [11,12]:
- Carcinoma
- End-stage interstitial cystitis
- Tuberculosis
- Ketamine bladder
- Unreconstructable vesico-vaginal fistula
Finally, a Mitrofanoff channel into native bladder when combined with bladder neck closure or into a heterotopic neobladder may offer symptom control in both paediatric and adults with end-stage incontinence who have failed to respond to multiple conventional surgical interventions [13].
A Mitrofanoff channel is often constructed simultaneously with other forms of lower urinary tract reconstructions such as heterotopic neobladder and enterocystoplasty, in order to allow continent storage and volitional emptying of urine. The MACE has somewhat gone out of surgical fashion but may be used to effect faecal continence in patients with neuropathic bowel dysfunction (spinal dysraphism, cloacal exstrophy and imperforate anus).
Preoperative work-up
All patients being considered for a Mitrofanoff procedure should be discussed in a multidisciplinary team (MDT) meeting and require input from a range of healthcare professionals to assess dexterity, patient motivation, psychosocial status and trajectory of any pre-existing neurological disease. Nutritional status, liver function and existing co-morbid conditions should be optimised preoperatively. If the Mitrofanoff channel is to be anastomosed into a native bladder it is imperative that the bladder is compliant with low pressure filling and therefore video urodynamics are an essential part of preoperative planning. Patients with a history of inflammatory bowel disease and pelvic radiotherapy are relative contraindications for the Mitroffanoff procedure as these can threaten the viability of the anastomosis and long-term function of the channel.
Patients and care-givers (if the patient’s upper limb function is impaired) should be educated and motivated to perform clean self-intermittent catheterisation via the channel. In addition, they should be aware of the potential complications of the procedure, the need for long-term follow-up and, in particular, the requirement for revision of the Mitrofanoff channel.
Contraindications
The patient must have sufficient manual and mental dexterity to perform stomal catheterisation and understand the need for life-long follow-up, and the pros and cons of the procedure and the alternatives. They do not however require as much dexterity and mobility as is required to undertake urethral intermittent self-catheterisation (ISC) and the Mitrofanoff technique has been utilised with good effect in quadriplegic patients. Patients must be highly motivated in order to undertake a lifetime of ISC +/- MACE catheterisation and to cope with the revision surgery that is often required.
The appendix is generally the conduit of choice but may be unavailable secondary to: appendicectomy, stenosis, short length, short mesentery or congenital absence. The advantages of the Yang / Monti ileal channel are: availability, generally easy to mobilise, reliable vascularity, and only a small (2.5cm) segment is required [3].
Consent
Consent is a dynamic process involving the doctor, the patient and the other members of the multidisciplinary team. If a patient is being considered for a Mitrofanoff procedure this should be agreed by the MDT. This then begins a process of counselling and educating as outlined above. As part of the consenting process the patient should be offered all viable alternative surgical options. It may take several outpatient sessions to complete the consent process. Once the patient is consented appropriately and judged motivated and educated sufficiently, a date for surgery can be set. Consent is finalised on the day of surgery. As part of the discussion the following risks should be outlined (risk percentages as per current BAUS figures) [14,15]:
- Generic complications of major abdominal surgery – wound infection / dehiscence / hernia (2-10%), bleeding (requiring blood transfusion / drainage of haematoma) (2-10%), ileus, venous thromboembolism, anaesthetic complications (0.4-2%).
- Complications associated with concomitant cystectomy or bladder reconstruction – anastomotic leak (2-10%), urine leak (2-10%), bowel obstruction (0.4-2%), enterocystoplasty or neobladder rupture (2-10%), ureteric anastomotic stricture (0.4-2%), metabolic complications (if enterocystoplasty or neobladder) (0.4-10%), long-term increased risk of cancer (enterocystoplasty) (0.4-1%).
- Complications specific to the Mitrofanoff procedure – stenosis (skin level / channel / anastomosis to bladder) requiring revision (2-10%), anastomotic leak (bowel and Mitrofanoff-bladder) (10%), incontinent channel (2-10%), false passage (0.4-2%), difficult catheterisation (0.4-2%), stomal prolapse.
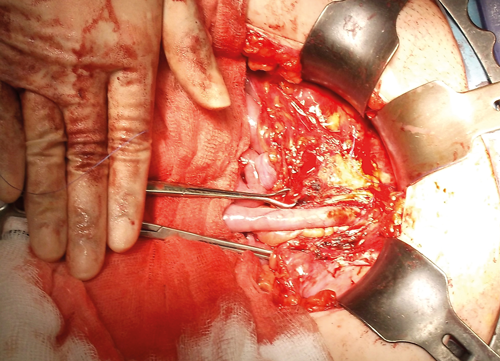
Figure 1. Mitrofanoff channel in situ.
Operative technique
Mitrofanoff channel formation
A Pfannenstiel or lower midline incision is used to gain access to the bladder and appendix / caecum although advances are being made in minimally invasive approaches. The appendix is mobilised on its mesentery and disconnected from the caecum (Figure 1). A 14-16Ch catheter is passed through the appendix channel to ensure it is patent and catheterisable. The reservoir end of the appendix is tunnelled into the bladder through a 3-4cm submucosal anti-reflux channel and anastomosed to the bladder urothelium. The appendix may be anastomosed posteriorly (studies have reported a higher rate of urinary tract infections and stones with anterior anastomosis) or anteriorly (which allows for a shorter channel and less catheterisation difficulties) [16]. The experience of the authors is that it is best to anastomose the appendix in the position which affords the shortest and most direct and straight channel.
Reservoir considerations
The bladder must be a low pressure reservoir for success and safety of the procedure and therefore additional augmentation ileocystoplasty is concurrently performed in some patients. Equally some patients will have undergone urethrectomy and / or cystectomy for carcinoma or other indications and will therefore have a neobladder as the reservoir.
Fortunately there appears to be similar rates of continence and requirement for future revision operations between Mitrofanoff channels implanted in native bladder versus bowel augment or neobladder providing a tunnelled anti-reflux technique is utilised [17]. The surgeon can choose the best functional position to implant the Mitrofanoff channel without being concerned about avoiding intestinal augmented sections.
Conversely, in order to achieve continence, some patients with large volume bladders may require additional procedures to achieve urethral continence ranging from bulking agents and mid-urethral slings to artificial urinary sphincters and bladder neck closure.
Stoma formation
There are various techniques described for creating the stoma. It should be sited in the lower abdomen, away from any scar sites. It is important to ensure that the appendiceal mucosa is not exposed at skin level (in contrast to the ileal conduit) as this can lead to complications with bleeding during clean intermittent self-catheterisation (CISC) and is cosmetically displeasing. Modern techniques aim to provide a short length of skin conduit before anastomosing to the appendix channel. Most commonly this is achieved at the umbilicus. The umbilicus is detached from the rectus sheath and a flap is created. The intestinal conduit is spatulated on its posterior surface and anastomosed to the umbilical flap. Similar techniques such as the tubularised skin flap and the VQZ flap have also been utilised if the stoma needs to be sited away from the umbilicus [18].
Postoperative care
Postoperatively patients are left with a 12Ch or 14Ch catheter in situ, often alongside a urethral or suprapubic catheter, for four to six weeks until the anastomosis has healed. They are brought back and taught to CISC through the Mitrofanoff channel and once established on this the urethral / suprapubic catheter can be safely removed.
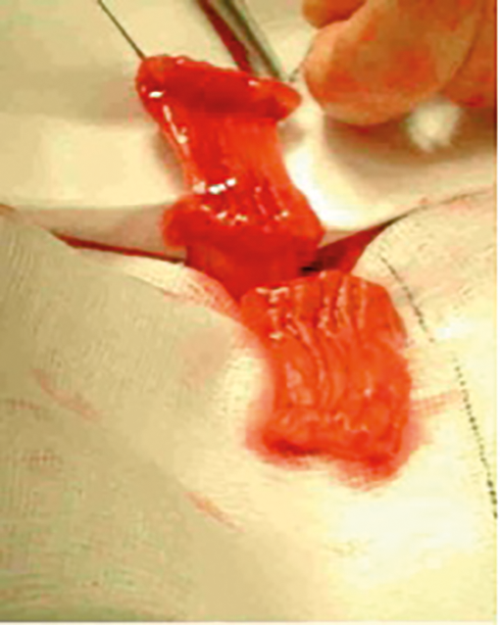
Figure 2a. Double monti harvest.
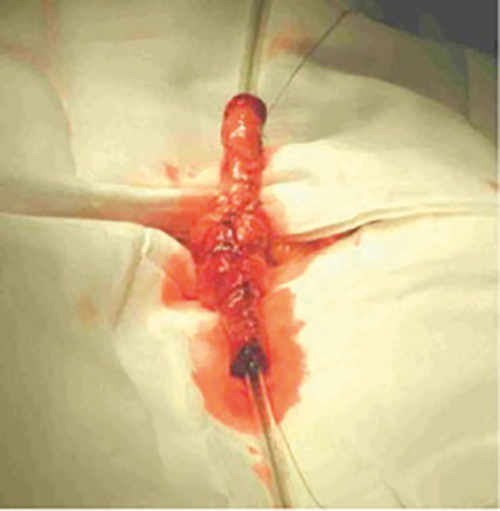
Figure 2b. Double monti complete.
Alternative options and surgical variations
Yang-Monti technique
One alternative to the appendix that has shown equivalent results is the Yang-Monti procedure and can be used when the appendix is absent, of insufficient length, or has a poor blood supply. This procedure isolates a 2-2.5cm length of ileum which is opened along its anti-mesenteric border and is then re-tubularised along its longitudinal axis in order to form a viable channel (Figure 2). When required this technique can be adapted to increase the length of the channel in a double Monti procedure in which two segments of bowel are isolated, de-tubularised and then joined together transversely. The resulting bowel plate is then tubularised to form a longer conduit. A spiral Monti uses a 3.5cm segment of isolated ileum. It is partially transected in the centre. Resultant segments are detubularised by incisions close to the mesentery on opposite sides. The flap is retubularised transversely to produce the conduit.
Ileum is readily available, easy to mobilise and produces conduits of good diameter and length. Resultant Yang-Monti channels are therefore commonly used and convey continence to over 95% of patients [19]. Such outcomes rival the traditional Mitrofanoff conduit. However, Monti stenosis rates are reported as 5-10% and revision rates two to four times more common in double and spiral Monti channels, respectively, relative to the traditional Mitrofanoff conduit [20]. Of note, a high incidence of diverticular pouches within the double Monti channel may account for difficult catheterisation and failure of the conduit [20]. A single ileal channel is therefore preferable to double and spiral Monti conduits where possible.
Long-term management and outcomes
General complications of the procedure include an early re-operative rate for significant postoperative complications requiring laparotomy of 8% [8]. The risk of long-term recurrent urinary tract infections and stone formation appears to be reduced with posterior implantation of the appendix channel into the reservoir. However, the urinary tract infection risk long-term ranges from 9.5-40% and stone formation of 40% [16,21]. The incidence of both conditions is obviously increased in patients with concomitant augmentation cystoplasty / neobladder.
The great attraction of this procedure is the longevity of continence with studies reporting continence ranging from 88-98% at medium to long term follow-up [9,17,22]. However, traditionally the major cause for re-operation involves problems with catheterising the channel including stenosis and false passages. Stomal stenosis is reported in up to 13% at two years and as high as 54% at five years [11,23]. However, in many, this can be managed conservatively with channel dilation or simple endoscopic procedures. Bladder level stenosis is rarer but often requires surgical intervention with excision of the stenosed segment, lengthening of the channel (if required) and repeat tunnelling and re-anastomosis. Other complications concerning the Mitrofanoff channel include difficulty with intermittent self-catheterisation, quoted at up to 20.1%, and stomal prolapse quoted at 2% [23,24].
Revision rates of the Mitrofanoff channel are reported at up to 20% for any indication [9,17]. Re-operation rates (early and late complications) total up to 32% [23]. There appears to be a benefit in terms of stomal stenosis with VQ, VQQ or VQZ plasties but the umbilicus is often favoured over these techniques due to superior cosmesis [25,26].
Comparisons between Mitrofanoff and Yang-Monti channels have yielded conflicting results. Some authors have shown no difference in the incidence of complications between appendicovesicostomy and ileovesicostomy [27,28]. Narayanaswamy et al., however found that Yang-Monti channels were more likely to have difficulty catheterising (60% of Yang-Monti vs. 26% Mitrofanoff) but that this was only due to stenosis in half of all cases in the Yang-Monti cohort. The remainder often had problems with a “pouch” in the channel or simply the channel was too long. Therefore a single Yang-Monti is recommended, where possible, compared to a double or spiral Monti [20].
Overall complications are common and re-operation rates high. However, this procedure has been shown to be well-tolerated and durable in the long-term despite the need for revision surgery. The disadvantages of a Mitrofanoff procedure are outweighed for many patients by the high continence rates, improvement in quality of life and cosmesis of the procedure.
Conclusion
Any patient undergoing a Mitrofanoff or Yang-Monti procedure must accept that they will inevitably require long-term follow-up, particularly if bladder reconstruction was also performed. The rate of complications and re-operation is high and patients must be counselled, in particular, regarding the risks of stomal stenosis and difficulties catheterising the channel requiring subsequent revision surgery. However, for patients with end-stage incontinence, neurogenic / end-stage bladder dysfunction or disrupted / non-functional urethrae, the Mitrofanoff procedure has provided a continent urinary conduit which is well tolerated and provides a good quality of life for our patients.
Almost 40 years since he first described this procedure, the Mitrofanoff procedure continues to show superiority to other continent conduits with the exception, perhaps, of the Yang-Monti conduit and is likely to continue to stay as an essential part of the armamentarium of functional, reconstructive and paediatric urologists for many years to come.
References
1. Mitrofanoff P. [Trans-appendicular continent cystostomy in the management of the neurogenic bladder]. Chir Pediatr 1980;21(4):297-305.
2. Yang WH. Yang needle tunneling technique in creating antireflux and continent mechanisms. J Urol 1993;150(3):830-4.
3. Monti PR, Lara RC, Dutra MA, de Carvalho JR. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology 1997;49(1):112‑5.
4. Jung P, Jakse G. The choice of continence mechanism in continent (supra)vesical urinary diversion. Urol Int 1996;57(3):175-9.
5. Bihrle R, Adams MC, Foster RS. Adaptations of the Mitrofanoff principle in adult continent urinary reservoirs. Tech Urol 1995;1(2):94-101.
6. Malone PS, Ransley PG, Kiely EM. Preliminary report: the antegrade continence enema. Lancet 1990;336(8725):1217-8.
7. Wedderburn A, Lee RS, Denny A, et al. Synchronous bladder reconstruction and antegrade continence enema. J Urol 2001;165(6 Pt 2):2392-3.
8. Gowda BDR, Agrawal V, Harrison SCW. The continent, catheterizable abdominal conduit in adult urological practice. BJU Int 2008;102(11):1688-92.
9. Harris CF, Cooper CS, Hutcheson JC, Snyder HM 3rd. Appendicovesicostomy: the mitrofanoff procedure-a 15-year perspective. J Urol 2000;163(6):1922-6.
10. Liard A, Seguier-Lipszyc E, Mathiot A, Mitrofanoff P. The Mitrofanoff procedure: 20 years later. J Urol 2001;165(6 Pt 2):2394-8.
11. Sahadevan K, Pickard RS, Neal DE, Hasan TS. Is continent diversion using the Mitrofanoff principle a viable long-term option for adults requiring bladder replacement? BJU Int 2008;102(2):236-40.
12. Ordorica R. The continent bladder: indications and techniques for the continent catheterizable segment. Curr Opin Urol 2004;14(6):345-50.
13. Woodhouse CR, Gordon EM. The Mitrofanoff principle for urethral failure. Br J Urol 1994;73(1):55-60.
14. BAUS. Enlargement of the bladder with a piece of Bowel (Enterocystoplasty) : Information about your procedure from The British Association of Urological Surgeons (BAUS). 2017.
www.baus.org.uk/_userfiles/
pages/files/Patients/Leaflets/
Enterocystoplasty.pdf
Accessed 26 November 2017.
15. BAUS. Mitrofanoff procedure (Creation of a catheterisable urinary stoma): Information about your procedure from The British Association of Urological Surgeons (BAUS). 2017.
www.baus.org.uk/_userfiles/
pages/files/Patients/Leaflets/
Mitrofanoff.pdf
Accessed 26 November 26 2017
16. Berkowitz J, North AC, Tripp R, et al. Mitrofanoff continent catheterizable conduits: top down or bottom up? J Pediatr Urol 2009;5(2):122-5.
17. Franc-Guimond J, Gonzalez R. Effectiveness of implanting catheterizable channels into intestinal segments. J Pediatr Urol 2006;2(1):31-3.
18. Woodhouse C. Continent Urinary Diversion. In: Frank J, Gearhart J, Snyder H, eds. Operative Pediatric Urology. Churchill Livingstone; 2002:79-108.
19. Wagner M, Bayne A, Daneshmand S. Application of the Yang-Monti channel in adult continent cutaneous urinary diversion. Urology 2008;72(4):828-31.
20. Narayanaswamy B, Wilcox DT, Cuckow PM, et al. The Yang-Monti ileovesicostomy: a problematic channel? BJU Int 2001;87(9):861-5.
21. Fishwick JE, Gough DC, O’Flynn KJ. The Mitrofanoff procedure: does it last? BJU Int 2000;85(4):496-7.
22. Thomas JC, Dietrich MS, Trusler L, et al. Continent catheterizable channels and the timing of their complications. J Urol 2006;176(4 Pt 2):1816-20.
23. Jacobson DL, Thomas JC, Pope J 4th, et al. Update on Continent Catheterizable Channels and the Timing of their Complications. J Urol 2017;197(3 Pt 2):871-6.
24. Suzer O, Vates TS, Freedman AL, et al. Results of the Mitrofanoff procedure in urinary tract reconstruction in children. Br J Urol 1997;79(2):279-82.
25. Kajbafzadeh AM, Chubak N. Simultaneous Malone antegrade continent enema and Mitrofanoff principle using the divided appendix: report of a new technique for prevention of stoma complications. J Urol 2001;165(6 Pt 2):2404-9.
26. Van Savage JG, Khoury AE, McLorie GA, Churchill BM. Outcome analysis of Mitrofanoff principle applications using appendix and ureter to umbilical and lower quadrant stomal sites. J Urol 1996;156(5):1794-7.
27. Lemelle JL, Simo AK, Schmitt M. Comparative study of the Yang-Monti channel and appendix for continent diversion in the Mitrofanoff and Malone principles. J Urol 2004;172(5 Pt 1):1907-10.
28. Castellan MA, Gosalbez R, Labbie A, et al. Outcomes of continent catheterizable stomas for urinary and fecal incontinence: comparison among different tissue options. BJU Int 2005;95(7):1053-7.
TAKE HOME MESSAGE
-
The Mitrofanoff procedure creates a continent appendicovesicostomy for patients with disrupted / non-functional urethrae, end-stage incontinence / detrusor overactivity or after cystectomy for benign or malignant causes.
-
If a patient is having a concurrent MACE and Mitrofanoff procedure the appendix should be used for the MACE procedure and a Monti substitution for the bladder conduit.
-
The Yang-Monti procedure is a suitable variation for patients with an absent or non-utilisable appendix but may have slightly higher complication rates.
-
Patients must be closely counselled about the risks of channel complications and the need for future revision surgery.
-
Continence rates are excellent and despite the high revision rates the procedure is well-accepted by patients
Declaration of competing interests: None declared.