Background
Urinary incontinence (UI) is a common symptom that can affect women of all ages. It is difficult to estimate the prevalence of UI since it is often under-reported, although the Norwegian EPINCONT study looking at women over 20 reported 25% of women suffering from urinary leakage and 7% having significant UI [1]. UI can cause a great deal of distress and embarrassment to women, which can have a detrimental effect on their physical, psychological and social wellbeing.
The National Institute for Health & Care Excellence (NICE) clinical guidelines on the management of UI in women provides evidence-based recommendations that can act as an informative tool for physicians in both primary and secondary care. The updated 2013 guideline acts to re-enforce the original guideline published in 2006 as well as incorporating recent evidence on increasingly popular treatments such as Botulinum toxin A and synthetic mid-urethral tapes. In this article we aim to summarise salient aspects of the guideline to increase awareness amongst clinicians.
Assessment and investigation
History taking
A thorough history should be the first step in assessing any patient with UI in order to formulate a clinical diagnosis. This includes a detailed urinary history to determine storage and voiding patterns and symptoms. The history should also identify accompanying symptoms that may indicate the possibility of a more serious diagnosis, such as haematuria, persisting bladder or urethral pain, or recurrent urinary tract infection (UTI). If such symptoms are elicited then an urgent speciality referral should be made. Other important aspects of a UI focused history relate to bowel symptoms, current medications, and a detailed past medical, surgical and obstetric and gynaecological history. An equally important area is the effect of UI on the individual patient in terms of social and functional impact.
Figure 1: Definitions (International Continence Society) [2].
Urinary incontinence (UI): The complaint of any involuntary loss of urine.
Stress UI: Involuntary urine leakage on effort or exertion, or on sneezing or coughing.
Urgency UI: Involuntary urine leakage accompanied by or immediately preceded by urgency.
Mixed UI: Involuntary urine leakage associated with both urgency and exertion, effort, sneezing or coughing.
Overactive bladder (OAB): Urgency that occurs with or without urgency UI and usually with frequency and nocturia. OAB that occurs with incontinence is known as ‘OAB wet’. OAB that occurs without incontinence is known as ‘OAB dry’.
Physical examination
Physical examination should include an abdominal examination to detect an enlarged bladder or a palpable pelvic mass. It also includes a vaginal examination to assess oestrogen status as well as pelvic organ prolapse. Routine assessment of pelvic floor muscle contraction is also recommended using grading scales such as the Oxford Grading System to quantify the strength of contraction.
Initial investigations
All women presenting with UI should have a urine dipstick and subsequent midstream urine specimen for culture if symptomatic or if urine dipstick is positive for either leucocytes or nitrites. Bladder diaries (minimum duration of three days covering variation in their activities) and quality-of-life assessments such as International Consultation on Incontinence Questionnaire (ICIQ) should also be provided in the first consultation. Traditional tests of urethral competence such as Q-tip are no longer advised. Cystoscopy and routine imaging should not be used in initial assessment of women with UI although ultrasound can be used to determine bladder post-void residual volumes.
Urodynamics
Urodynamics testing encompasses a number of physiological tests of bladder and urethral function. The term is often used in reference to multi-channel cystometry. The NICE guideline recommends that multi-channel cystometry should be used before proceeding to surgery and also reviewed in multidisciplinary team (MDT) meetings for women with symptoms that are suggestive of detrusor overactivity; symptoms suggestive of voiding dysfunction or anterior compartment prolapse; or those who have had previous surgery for SUI. Urodynamics is not recommended where pure stress UI is diagnosed without any urge symptoms [3].
After clinical assessment, the clinician should aim to categorise the woman’s UI as stress UI, urgency UI / overactive bladder (OAB) or mixed UI. Initial treatment should be started on the basis of this clinical diagnosis. In mixed UI, treatment should be directed towards the predominant symptom.
Conservative treatment
Lifestyle interventions
Often the initial lifestyle intervention encouraged is a trial of caffeine reduction. A randomised controlled trial suggested that reduction in caffeine may improve symptoms of urgency and frequency though does not improve UI [4]. Other interventions include modification of fluid intake and encouraging weight loss in women with a BMI greater than 30.
Physical therapies
Pelvic floor muscle training (PFMT) is a technique used to increase strength and durability of contraction of the pelvic floor muscles. This increases urethral closure pressure and stabilises the urethra, preventing downward movement during increased activity. The guideline recommends supervised PFMT of at least three months’ duration as first-line treatment for women with stress or mixed UI. It is also recommended that PFMT programmes should comprise at least eight contractions performed three times per day.
Figure 2: Cost of antimuscarinics.
Antimuscarinic: Oxybutynin (immediate release)
Cost of a month’s supply: £1.06 - £1.76
Antimuscarinic: Tolterodine (immediate release)
Cost of a month’s supply: £1.18
Antimuscarini: Darifenacin
Cost of a month’s supply: £30.58
Antimuscarini: Trospium chloride XL 60mg
Cost of a month’s supply: £23
Antimuscarini: Fesoterodine 4 and 8mg
Cost of a month’s supply: £28.62
Antimuscarini: Solifenacin 5 and 10mg
Cost of a month’s supply: £29.70 - £38.64
Bladder training
Bladder training actively involves the patient, in attempting to increase the interval between the desire to void and the actual void [5]. Bladder training lasting a minimum of six weeks should be offered as first-line treatment to women with urgency or mixed UI. If women are not seen to achieve satisfactory benefit from bladder training then it can be considered in combination with pharmacological therapy.
Alternative conservative options
Absorbent products, hand held urinals and toileting aids should not be considered as routine treatment options for UI but instead as a coping strategy pending definitive treatment. It can also be used as an adjunct to ongoing therapy. Intermittent or indwelling urethral catheters should only be considered in women in whom persistent urinary retention is causing UI and when this can either not be corrected or it is the patient’s preference to have a catheter. Intravaginal and intraurethral devices are also not routinely recommended in the treatment of UI apart from when being used as a preventative measure, for example during exercise.
Pharmacological treatment
If conservative management is not beneficial in the treatment of OAB or mixed UI then pharmacological treatment is considered. Antimuscarinic drugs have traditionally formed the mainstay of treatment of OAB / mixed UI. It is important that a patient is counselled regarding the adverse effects including dry mouth and constipation. New additions to the 2013 guideline include prescribing the lowest recommended dose when starting a new drug treatment as well as informing the patient that full benefits may not be seen until four weeks. At present the current first-line treatment options based on cost-effectiveness are oxybutynin (immediate release), tolterodine (immediate release) or darifenacin (once daily preparation). If however, this first-line treatment is not effective or not well-tolerated then NICE states that another drug with the lowest acquisition cost should be used.
In our unit, women are offered trospium chloride, fesoterodine or solifenacin as second-line therapy. Darifenacin is not offered as a first-line option as it is not of lowest acquisition cost and there is limited local clinical experience. Figure 2 states the cost of a month’s supply of the common antimuscarinics at our trust.
Oxybutynin (immediate release) should be avoided in frail older women as there is some evidence that it can worsen cognitive dysfunction. In June 2013, NICE offered technology appraisal guidance on the drug Mirabegron (beta-3 adrenoreceptor agonist) and recommends its use if antimuscarinics are not beneficial or the side-effects are unacceptable.
It is important that OAB drugs are reviewed after being initiated. Given the lag period of approximately four weeks in most patients before effects are seen, a telephone or face-to-face review is advised after four weeks to assess satisfaction and response to therapy. Women on long-term drug treatment should be reviewed annually in primary care (or every six months for women over 75).
If drug treatment is unsuccessful or the patient wishes to discuss further management options then she should be referred to secondary care.
Figure 3: Multidisciplinary team for urinary incontinence.
-
A uro-gynaecologist
-
A urologist with sub-specialist interest in female urology
-
A specialist nurse
-
A specialist physiotherapist
-
A colorectal surgeon
-
A member of care of the elderly team and / or occupational therapist for women with functional impairment.
Desmopressin is a modified version of the human hormone arginine vasopressin. It increases water reabsorption by acting on V2 receptors in the renal collecting ducts. It can be considered specifically to reduce nocturia in women with UI or OAB. It is to be avoided in women above 65 years who have cardiovascular disease or hypertension due to risk of hyponatraemia. Duloxetine is a serotonin and noradrenaline reuptake inhibitor (SNRI) and should not be used routinely in the treatment of stress UI. It may be considered if the patient prefers pharmacological therapy to surgery or is not suitable for surgical intervention.
Intravaginal oestrogens can be offered for the treatment of OAB in post-menopausal women with vaginal atrophy.
In the updated 2013 NICE guideline it is recommended that invasive treatments including surgery for OAB and / or SUI symptoms should only be offered after MDT review. Figure 3 outlines those included in the MDT team.
Invasive treatments for overactive bladder
Botulinum toxin A
Botulinum toxin A can be offered to women with proven urodynamic detrusor overactivity that have not responded to conservative and pharmacological therapy. It is important that patients are counselled about adverse effects including urinary tract infections and urinary retention. It is therefore very important that patients are taught and are confident in performing clean intermittent catheterisation before the treatment is started. They should also be aware that should the treatment be successful, there is a variable duration of effect and furthermore that as it is a relatively contemporary technique, long-term data on efficacy and risks are not currently known.
A dilemma for clinicians remains the dose of botulinum toxin A to prescribe. The guideline recommends 200 units as an initial dose though mentions that 100 units can be considered for those who would prefer a lower chance of having to perform intermittent catheterisation and would accept a reduced chance of success. It is important for clinicians to also be aware that different manufacturers may have different dose strengths that they recommend. Of interest, the European Association of Urology Guidelines on Urinary Incontinence 2013 states that 100 units should be used as the first dose in order to minimise the risk of urinary tract infection and retention [6]. Allergan’s botulinum toxin A became licensed in the UK in September 2013 for use in patients with overactive bladder. The licensed dose was 100 units in idiopathic OAB and 200 units in neuropathic OAB [7].
With further research on the topic, it is hoped that in the future more information will be available on appropriate initial and follow-up doses as well as the most effective method of injecting botulinum toxin A in terms of site of injections, number of injections and long-term outcomes.
Neurostimulation
Techniques using electrical stimuli directed at the sacral nerve roots have been adopted as therapy for OAB. The principle is that electric stimulation of sacral pathways will inhibit the reflex behaviour of the bladder and reduce detrusor overactivty. These techniques include transcutaneous sacral nerve stimulation (t-SNS) where surface electrodes are placed above the sacrum, transcutaneous posterior tibial nerve stimulation (t-PTNS) with surface electrodes above the posterior tibial nerve and percutaneous posterior tibial nerve stimulation (p-PTNS) where a needle is inserted close to the posterior tibial nerve. The guideline suggests there is insufficient evidence to recommend either t-SNS or t-PTNS to treat OAB. The updated guideline suggest p-PTNS should not be used routinely to treat OAB unless conservative management including OAB drug treatment has not worked and the patient does not want either Botulinum toxin A or percutaneous sacral nerve stimulation.
“If the first-line anti-muscarinic is not effective or not well-tolerated then an anti-muscarinic with the lowest acquisition cost should be used.”
When considering percutaneous sacral nerve stimulation, patients first undergo test stimulation where a needle is inserted through the sacral foramina under local anaesthetic and then connected to an external stimulation source. If there is a satisfactory response to the test simulation then a permanent implant can be offered. This treatment should be offered once again only after MDT review if a woman’s OAB has not responded to conservative treatment, pharmacological therapy and botulinum toxin A. It can also be considered if a patient is unable to perform clean intermittent catheterisation and therefore unable to proceed with botulinum toxin A. The patient should be counselled regarding the need for test stimulation, risk of failure, long-term commitment, need for surgical revision and adverse effects.
Augmentation cystoplasty and urinary diversion
Augmentation cystoplasty should be restricted for the management of idiopathic detrusor overactivity when patients have not responded to conservative treatments and are able to self-catheterise. Complications should be discussed including bowel disturbance, metabolic acidosis, mucus production, UTI, urinary retention and the small risk of malignancy occurring in the augmented bladder. Life-long follow-up needs to be provided.
Urinary diversion should only be considered when all conservative and other invasive therapies have failed.
Surgical procedures for stress urinary incontinence
Surgical treatment is the standard approach for patients with SUI who have failed conservative therapy. Since the first report from the Ulmsten group, tension-free mid-urethral tapes have become one of the most commonly performed procedures worldwide [8,9]. It has also been shown to be very effective with an 11-year prospective study showing 90% of women treated were still objectively cured at last follow-up [10]. Mid-urethral tapes can be broadly divided into retropubic or transobturator approaches. The retropubic tape can be inserted from below or from above. The transobturator tapes can be inserted via an ‘inside-out’ technique or ‘outside-in’ approach. A recent systematic review and meta-analysis showed the subjective cure rates of retropubic tapes to be similar to the transobturator tapes [9]. However, transobturator tapes had a lower risk of bladder and vaginal perforations and storage lower urinary tract symptoms.
The guideline however notes the lack of long-term data with the transobturator approach and also that the ‘top-down’ retropubic approach can only be currently used as part of a clinical trial. The guideline emphasises the issues of safety for all mid-urethral tapes given its increasing popularity. This includes guidance on using only devices that a clinician has been trained to use and tapes always manufactured from type 1 macroporous polypropylene. Other safety recommendations are tapes coloured for high visibility for ease of insertion or revision. Patients should also be counselled about risks of tape erosion that may have effects in the long term that are yet to materialise. In the short term it is advised that within a six-month follow-up period, a vaginal examination is conducted to exclude erosion.
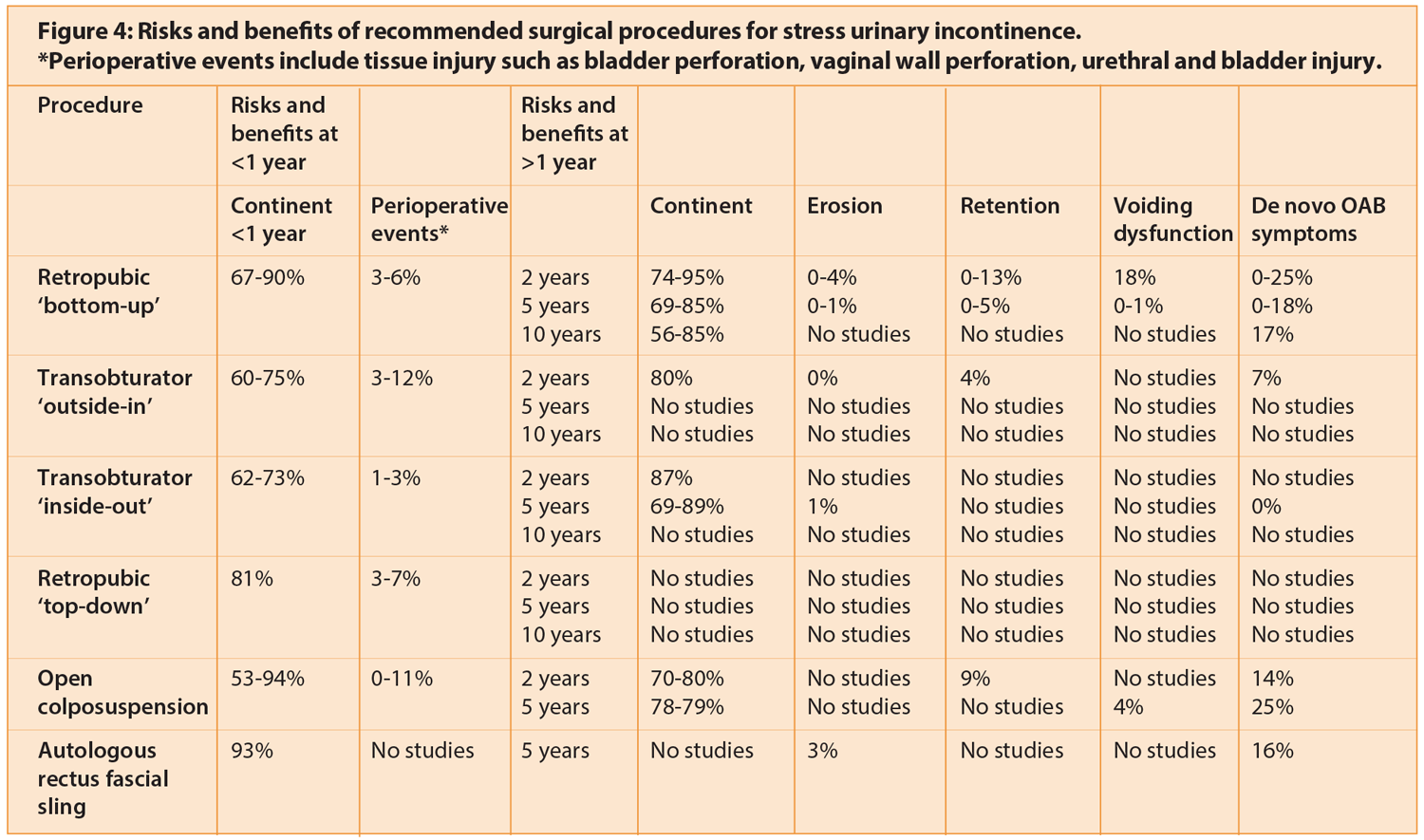
The open colposuspension aims to approximate the lateral tissue of the vaginal vault to the pectineal ligament by means of insertion of several non-absorbable sutures. Open colposuspension was previously the gold-standard surgical intervention for SUI but has become less commonly used with the increasing popularity of mid-urethral tapes. Autologous rectus fascial slings are recommended after failed conservative management of stress UI or in women who are not keen on the risks associated with synthetic tapes including erosion. It involves the use of a strip of the rectus sheath as a mid-urethral sling. Figure 4 outlines the risks and benefits of the different surgical procedures.
Intramural bulking agents work by creating artificial urethral cushions that improve UI. The agents are mainly collagen or silicone-based and are usually inserted under cystoscopic guidance peri-urethrally. It is a quick procedure with low perioperative complication rates but efficacy diminishes with time, therefore repeat injections are required. Efficacy of intramural bulking agents is inferior to that of synthetic tapes.
In view of the associated morbidity, the use of an artificial urinary sphincter should be considered for the management of stress UI in women only if previous surgery has failed. Life-long follow-up is recommended.
Conclusions
Urinary incontinence in women is a significant health issue. This updated NICE guideline provides comprehensive information on how women with urinary incontinence should be managed. The new recommendations in this updated version include the use of cost-effective antimuscarinics, the use of percutaneous posterior tibial nerve stimulation only after botulinum toxin A and percutaneous sacral nerve stimulation have been considered, and the use of percutaneous sacral nerve stimulation following non-response with botulinum toxin A. The importance of MDT review to ensure high quality care is also highlighted.
References
1. Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: The Norwegian EPINCONT Study. Journal of Clinical Epidemiology 2000;53(11):1150-7.
2. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation sub-committee of the International Continence Society. Urology 2003;61(1):37-49.
3. Nager CW, Brubaker L, Litman HJ, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med 2012;366(21):1987-97.
4. Bryant CM, Dowell CJ, Fairbrother G. Caffeine reduction education to improve urinary symptoms. British Journal of Nursing 2002;11(8):560-5.
5. Wallace SA, Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. (Cochrane Review). Cochrane Database of Systematic Reviews 2004;1.
6. European Association of Urology Guidelines on Urinary Incontinence 2013.
7. UK BOTOX Summary of Product Characteristics, 2013.
http://www.medicines.org.uk
Last accessed March 2014.
8. Ulmsten U, Henriksson L, Johnson P, Varhos G. An ambulatory surgical procedure under local anesthesia for treatment of female urinary incontinence. Int Urogynecol J 1996;7:81-6.
9. Novara G, Artibani W, Barber MD, et al. Updated Systematic Review and Meta-Analysis of the Comparative Data on Colposuspensions, Pubovaginal Slings, and Midurethral Tapes in the Surgical Treatment of Female Stress Urinary Incontinence. Eur Urol 2010;58(2):218-38.
10. Nilsson CG, Palva K, Rezapour M, Falconer C. Eleven years prospective follow-up of the tension-free vaginal tape procedure for treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1043-7.
TAKE HOME MESSAGE
-
Clinical history and examination form the cornerstone of identifying a clinical diagnosis of UI and type of UI.
-
Management of OAB:
- Lifestyle interventions and bladder training
- Antimuscarinics
- Mirabegron
- Botulinum toxin A
- Percutaneous sacral nerve stimulation
- Augmentation cystoplasty
- Urinary diversion.
-
Management of SUI:
- Pelvic floor muscle training
- Synthetic tapes / colposuspension / autologous rectus fascial sling
- Artificial urinary sphincter.
Declaration of competing interests: None declared.






