From the first cystoscopic argon and neodymium-YAG (yttrium-aluminium-garnet) laser used for bladder tumours in 1976 by Staehler et al. [1], lasers have proven to be a versatile and an evolving tool in the therapeutic management of a variety of urological diseases, including benign prostatic hyperplasia (BPH), kidney stone disease (KSD), urinary strictures, bladder and penile tumours [1-4].
Lasers have allowed minimisation of procedures to help safely treat an ageing population with multiple co-morbidities resulting in shortened anticoagulant interruptions, less invasive procedures and reduce hospitalisation time [2].
The application of lasers in endourology has broadened in the past three decades due to numerous advancements in laser technology, equipment, settings and the associated costs [3]. The need for thin and flexible yet durable and precise delivery systems has led to the development of specialised instruments for endourological surgery. Improvements and education of laser safety and related complications has increased the availability and usability of a variety of laser types and techniques; however, the high cost of newer laser technology can limit its more regular usage in urology departments. Here we look at the lasers currently used in endourology and take a sneak peek at the newcomers in this technologically developing field.
Type of lasers
All lasers consist of an energy source, an active medium, and a resonant cavity. The energy source simultaneously brings the atoms or molecules within the active medium to a higher energy state, referred to as pumping. The active medium of a laser is what determines its wavelength and frequency, and may be a solid, liquid or gas. Various active mediums used over the years have ranged from gases such as nitrogen, carbon dioxide, and helium to liquids such as neon dye lasers. The latter uses a solution containing a complex organic dye; the choice of dyes enables production of laser light over a broad range of wavelengths. Solid active mediums such as neodymium-doped yttrium-aluminium-garnet (YAG) were developed in the 1960s and are still in use today [4]. An additional internal or external accessory device can be added to convert the output to visible or ultraviolet wavelength. Semiconductor, also known as diode lasers, are made up of two semiconductor material layers. Despite being small and of low power, they can be combined to create larger, more powerful versions. Common endourology lasers include the Holmium (Ho:YAG), Potassium titanyl phosphate (KTP: YAG), commonly referred to as the ’green light’ laser, and Thulium (Tm:YAG and fiber (TFL)) lasers.
Controllable parameters
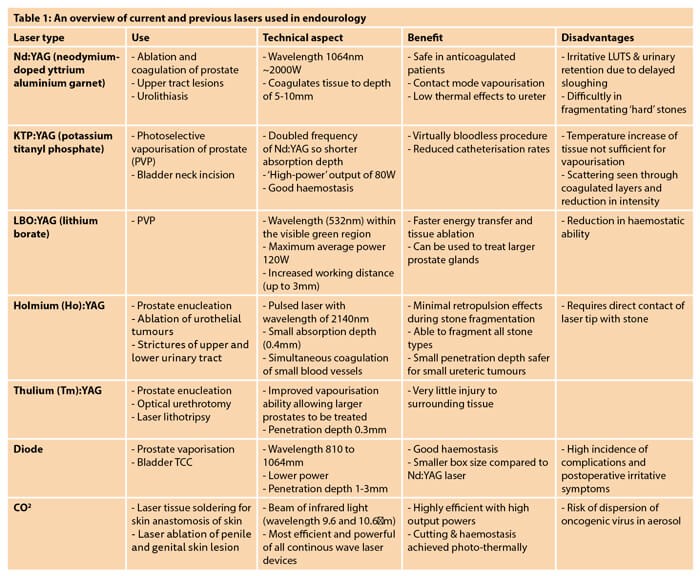
Settings such as pulse energy, frequency, width, power and fibre sizes are factors that can impact the efficacy and safety of a laser. Many studies have looked into understanding these technical factors and how they can be used to optimise the use of lasers and reduce complications. Other characteristics of different lasers that need to be taken into consideration include penetration depth, thermal effects and scattering of light energy. The amount of localised thermal damage, absorbed laser light and absorption depth of each laser is of clinical significance. This is a function of the wavelength and power density of the laser and the absorption coefficient of the tissue. An overview of currently used lasers, their application, benefits and disadvantages can be found in Table 1.
High power Ho:YAG and MOSES technology
The Ho:YAG laser is a multi-functional laser that has been reliably used for over 20 years in endourology. The 2140nm wavelength is strongly absorbed in water, which means it travels only about 0.3-0.5mm in a fluid medium, making it ideal for urological use within a limited space such as the ureter or renal pelvis. A higher pulse frequency Ho:YAG laser (HPL) resulting in a higher energy output (100/120W) has been studied for use in retrograde intrarenal surgery (RIRS) [5]. It can be used for stone fragmentation, dusting, pop-corning and pop-dusting by changing the energy and frequency settings (Table 2).
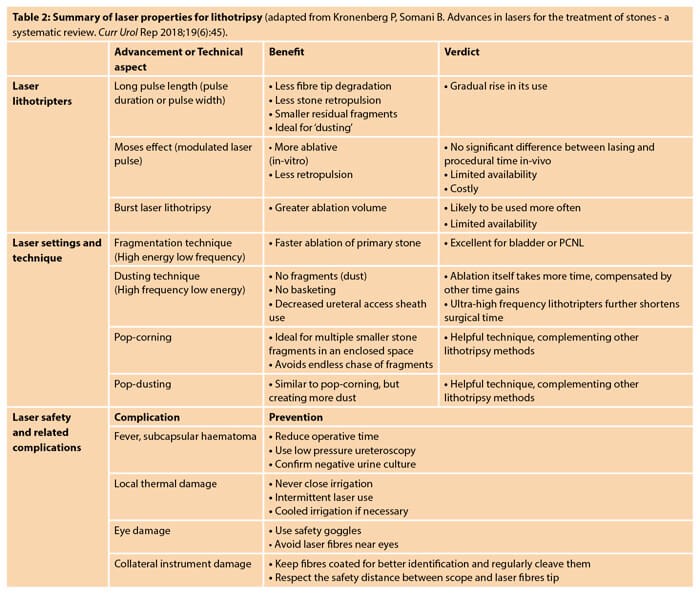
Whilst the high energy and low frequency settings are used for fragmentation, low energy and high frequency settings are used for dusting. In pop-dusting, a low energy beam in a contact mode is used first to break down the soft outer shell of a stone, then switching to a higher energy beam in a non-contact mode to ‘pop’ the hard core into dust, all of which can then be passed spontaneously. This method also reduces the number of treatments needed for large stones: in one study 93% of patients were stone-free after one treatment [6]. HPL is more expensive than the existing low-power Ho:YAG, which are the most commonly used in stone surgery currently, but this cost is likely to be balanced out by reducing the need for completion or secondary procedures.
Changing the settings such as pulse energy, frequency and pulse width can affect the power output of the laser and so it can be readily tailored towards different functions. A new dual-phase MOSES ‘pulse modulation’ technology has been introduced in high-power laser machines. Patented by Lumenis, MOSES pulse technology works to modulate the pulses into two peaks; the first separates the water and the second targets the stone through the bubble created by the first peak [7]. This aims to improve stone fragmentation without the increased stone retropulsion which is seen with high power Ho:YAG lasers, saving time and increasing efficiency. Studies have shown a 20% reduction in procedure time and a 50% reduction in stone retropulsion [7].
Thulium lasers
Thulium:YAG laser (Tm:YAG) is already established as an effective method of prostate vapourisation and enucleation. This differs from Ho:YAG in that the energy source which excites the thulium ions are from high-power laser diodes, which means there is less heat generation and increased power generation by a factor of five. Recent newer application of thulium laser technology has renewed interest in this for KSD. Super-pulsed thulium fibre lasers (TFL) use electronically modulated laser diodes allowing delivery of a higher and more constant peak power with a wider range of laser parameters through a fibre [8]. Water absorbs TFL energy approximately four times higher than Ho:YAG, and hence its optical penetration depth in water is four-times shorter than the Ho:YAG laser, resulting in lower stone and tissue ablation thresholds [9]. Smaller laser fibre sizes allow low pulse energy to be delivered with a very high pulse frequency thus minimising inadvertent surrounding tissue damage, giving this laser ideal physical properties for stone lithotripsy and prostate enucleation [10]. Studies are ongoing to fully understand the benefits as well as limitations of these lasers.
Laser fibres
The fibres themselves are an important and emerging concept in laser design. The need for thin, flexible lasers that don’t compromise on intensity or durability are vital in endourology. With miniaturisation of instruments, the diameter of fibres (made up of the fibre core and two to three outer layers) need to fit in a working channel alongside irrigation flow. The choice of outer layer cladding material (the majority are silica) determines the bending radius allowing for curvature. It can also affect the amount of undesirable attenuation and leakage resulting not only in reduction of power but damage to the scopes themselves. Wear and tear, whether inadvertently or due to excessive bending can cause radiation leakage as well as explosive fibre failure. The balance seems to be between a thin, flexible fibre which provides a higher laser intensity at the distal tip but is robust enough to prevent breakage and regular (costly!) replacement. Side firing tips are also available and offer an increased beam expansion at the tip and better control on the point of impact.
Conclusion
‘With great power comes great responsibility’ – increased tissue injury, more stone retropulsion, sub-optimal results, and a larger repair bill! The future of lasers in endourology isn’t all about power and recent studies have focused more on power delivery methods, pulse optimisation, techniques, equipment size and durability. The increased use of lasers on a day-to-day basis requires knowledge and clear understanding of how lasers work and how adjusting the settings of a laser can significantly change its characteristics to adapt it to specific urological applications. Lasers are one of the safest tools in endourology and newer developments in this technology will allow further minimisation and increased efficacy and safety of endourological procedures with exciting new developments in the future.
TAKE HOME MESSAGE
-
Lasers are an evolving and vital tool in the therapeutic management of a variety of urological diseases.
-
Most common procedures include intrarenal lithotripsy for kidney stone disease and ablation and vapourisation of the prostate in BPH.
-
High energy and low frequency settings are used for stone fragmentation; low energy and high frequency settings are used for stone dusting.
-
Ho:YAG laser is the current gold-standard for stone disease and with the addition of new dual-phase MOSES ‘pulse modulation’ technology it has improved stone fragmentation without increased stone retropulsion.
-
The newer thulium fibre lasers appear to be superior in terms of fragmentation speed, particle size, retropulsion distances and portability but currently lack supporting data from clinical trials.
References
1. Staehler G, Hofstetter A, Gorisch W, et al. Endoscopy in experimental urology using an argon-laser beam. Endoscopy 1976;8(1):1-4.
2. Dołowy Ł, Krajewski W, Dembowski J, et al. The role of lasers in modern urology. Cent European J Urol 2015;68(2):175-82.
3. Kronenberg P, Somani B. Advances in lasers for the treatment of stones – a systematic review. Curr Urol Rep 2018;19(6):45.
4. Teichmann HO, Herrmann TR, Bach T. Technical aspects of lasers in urology. World J Urol 2007;25:221-5.
5. Basulto-Martínez M, Proietti S, Yeow Y, et al. Holmium laser for RIRS. Watts are we doing? Arch Esp Urol 2020;73(8):735-44.
6. Pietropaolo A, Jones P, Whitehurst L, Somani BK. Role of ‘dusting and pop-dusting’ using a high-powered (100 W) laser machine in the treatment of large stones (≥ 15 mm): prospective outcomes over 16 months. Urolithiasis 2019;47(4):391-4.
7. Ibrahim A, Badaan S, Elhilali MM, Andonian S. Moses technology in a stone simulator. Can Urol Assoc J 2018;12(4):127-30.
8. Netsch C, Gross AJ, Herrmann TRW, Becker B. Current use of thulium lasers in endourology and future perspectives. Arch Esp Urol 2020;73(8):682-8.
9. Kronenberg P, Traxer O. The laser of the future: reality and expectations about the new thulium fiber laser-a systematic review. Transl Androl Urol 2019;8(Suppl 4):S398-S417.
10. Schembri M, Sahu J, Aboumarzouk O, et al. Thulium fiber laser: The new kid on the block. Turkish Journal of Urology 2020 [Epub ahead of print].
Declaration of competing interests: None declared.





