Klinefelter’s syndrome (KF) is the most frequent sex chromosome abnormality with an estimated prevalence of 1 in 500 to 1 in 700 newborn males and 1 in 10 in men with azoospermia. While the majority of cases are an XXY genotype, mosaicisms or other aneuploidies can be detected [1].
It is important to be aware of this syndrome within the urology clinic as it is the most common cause of inherited male hypogonadism, with significant effects on male fertility. Overall, it is seen in 3.1% of infertile males [2]. This article aims to review the etiology and epidemiology of KF as well as the disease’s implications on fertility.
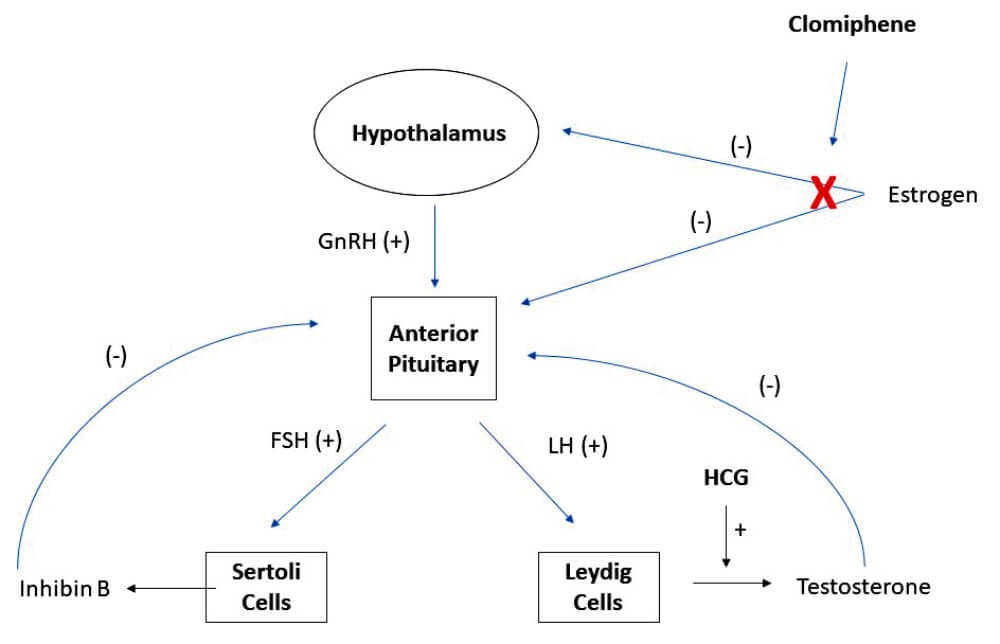
Figure 1: Hypothalamic–pituitary–gonadal (HPG) reproductive hormone axis.
Etiology and epidemiology
The origin of the XXY genotype is most commonly from errors related to nondisjunction in paternal meiosis 1 [3]. Approximately 15-20% of KS men are defined as ‘mosaics’, usually with two cell lines: 47,XXY / 46,XY [4]. Although, this figure may be underestimated due to different chromosomal mosaicism levels in different tissues. In addition, mosaic KS men are more androgenised than true XXY men which may result in under detection of men with mosaic KS [5].
In KF, testis biopsy shows Leydig cell hyperplasia, tubular hyalinisation, thickening of the basement membrane, and Sertoli-only cell phenotype [6]. Improper functioning of Leydig cells causes high estradiol levels and low to normal testosterone.
Unfortunately, KF is severely underdiagnosed with epidemiologic studies demonstrating that only 25% of patients are ever diagnosed and few of these before the onset of puberty [7]. This may be changing rapidly, however, as a recent study demonstrated the ease of KF testing with dried blood spot samples, potentially allowing simple and affordable newborn screening [8].
“We recommend close collaboration with a reproductive endocrinology specialist to hormonally optimise patients prior to surgical sperm retrieval”
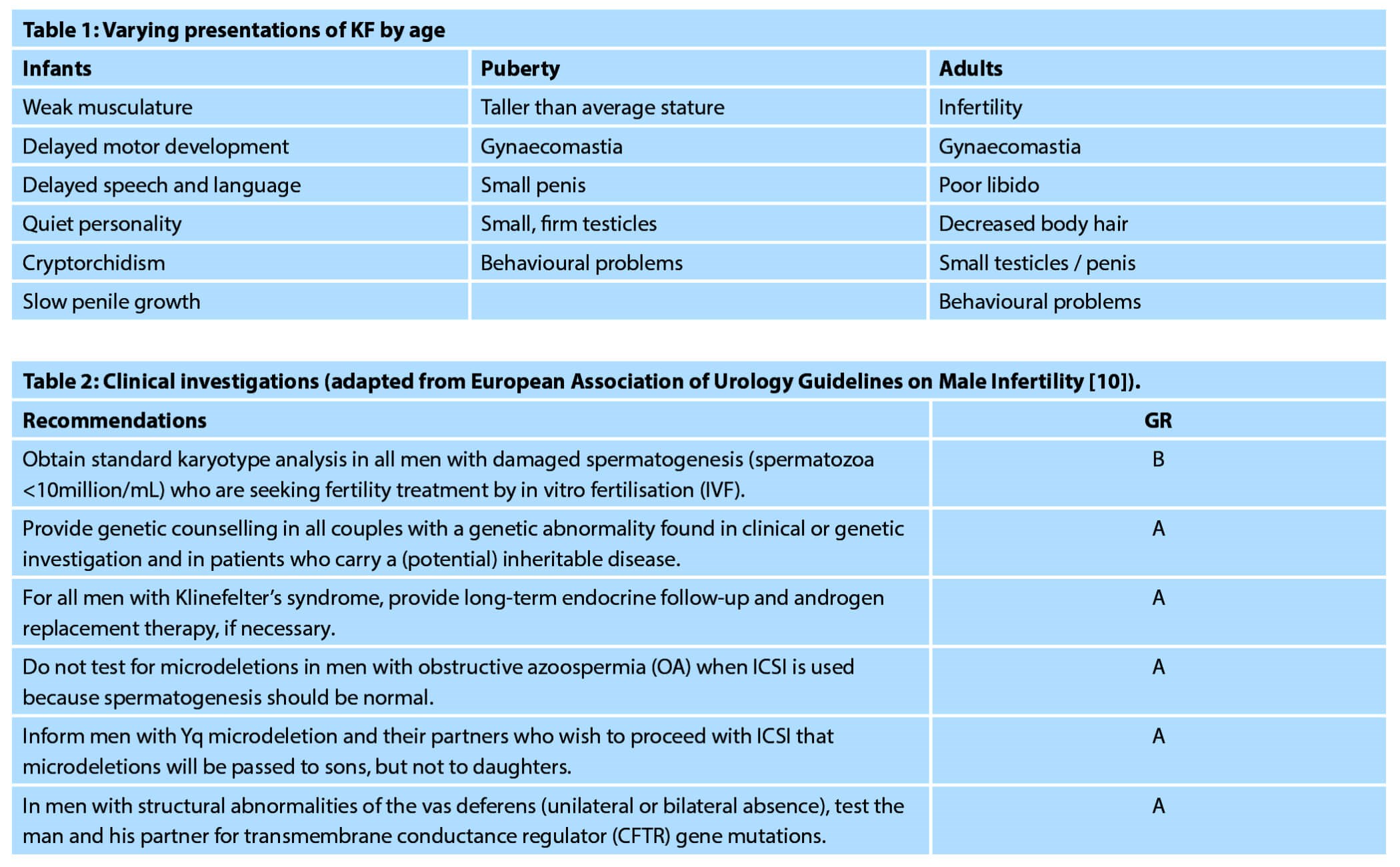
Men are often diagnosed at puberty or in adulthood because of minimal testicle growth or obvious gynaecomastia, however a common presentation will be in the urology clinic with a diagnosis of infertility and azoospermia [9]. In those cases, a full genetic work-up should be completed including karyotype, Y chromosome microdeletion test, and CF carrier status (if any abnormality of vas deferens is detected) [10]; hormones, including testosterone, follicle-stimulating hormone (FSH), and luteinising hormone (LH), should also be checked.
Clinical presentation
Signs and symptoms can differ in different age groups depending on the severity of the phenotype. Clinically, it is worth recognising these differences to aid in diagnosis. In early life, KF patients can present with weak musculature and delayed motor and speech development. While most boys have sufficient testosterone to initiate puberty, most fail to progress properly [11] and are noted to have small firm testes, small penis, and gynaecomastia. Undescended testes are more common in KF although there has been no data showing higher rates of testicular cancer in these patients [12]. In adulthood, patients present with infertility, gynaecomastia, poor libido, decreased body hair, and behavioural problems. Mosaic patients often present with milder phenotypes depending on their degree of mosaicism [1,13].
Sperm extraction – when, how, and will it be successful?
If the man is found to have very low levels of sperm in their ejaculate, there are reports in the literature of successful intracytoplasmic sperm injection (ICSI) using this ejaculated sperm [14,15] in KF men. However, these cases are rare and the majority of patients need surgical sperm retrieval to achieve pregnancy. Even if there is no sperm found with the ejaculate, small numbers of sperm may still be found within the testicle itself. Prior to 1996, when Tournaye et al. first reported successful recovery of sperm by testicular sperm extraction (TESE) in men with KF and azoospermia [16], these patients were considered definitively sterile. One year later, Palermo documented the first pregnancy using TESE / ICSI [17]. Multiple studies have now shown that despite widespread scarring of the seminiferous tubules, there are residual foci of spermatogenesis amenable to surgical sperm retrieval and assisted reproductive methods [13,18].
In traditional TESE, a small incision is made in the tunica albuginea of the testicle, seminiferous tubules are extracted, and the incision is closed. This method does not allow for targeting of any certain areas of the testis based on appearance. Many papers have shown high rates of sperm retrieval (47-69%) using microsurgical testicular sperm extraction (mTESE), which is a more invasive form of sperm retrieval in which the testicle is bivalved and the tubules examined directly with microscopic magnification [18-24].
The technique was first described in a seminal paper by Schlegel et al. in 1999 which showed a 63% sperm retrieval rate with mTESE as compared to 41% by standard TESE [25] in all cases of azoospermia. In 2009, Ramasamy and Schlegel demonstrated a 68% sperm retrieval rate using mTESE in KF patients [18]. Based on higher sperm retrieval rates, our institution utilises mTESE for all cases of testicular sperm extraction in KF patients. When the testicle is bivalved, it is common to see flat scarred-appearing tubules with small pockets of healthier-appearing tubules that are targeted for retrieval, as seen in Figure 2.
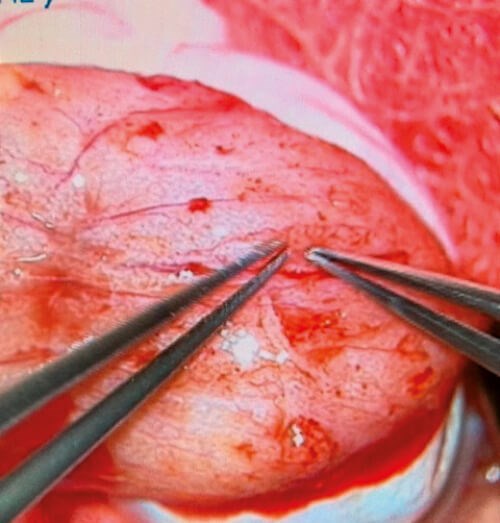
Figure 2: Seminierous tubules under the operative microscope in the testicle of a patient with KF.
Recent reviews have shown very wide-ranging success rates of sperm retrieval in KF (13-68%) with no convincing predictors of success. Analyses of age, testicle size, FSH, LH, or testosterone levels have not shown any significant association with success of sperm retrieval. Based on previous reports of progressive hyalinisation of seminiferous tubules in post pubertal KF patients, it has been suggested that performing earlier TESE may result in high sperm retrieval rates [26, 27]. However, a more recent meta-analysis from 2017 showed that increasing age was not associated with worsening sperm retrieval rate [28], which is consistent with our institutional series. Notably, numerous studies show comparable sperm retrieval, fertilisation, implantation, and live birth rates between KS and other non-obstructive azoospermia (NOA) patients [21,23,29]. A meta-analysis from 2017 showed that eventually, 16% of KF couples undergoing assisted reproductive techniques will have a child [28].
Hormonal manipulation
If men have been previously diagnosed with KS, they often attend their first infertility consultation receiving supplemental testosterone. This medication will suppress the production of spermatogenesis, thus gonadotrophins and semen analysis results will need to be interpreted accordingly.
While the use of testosterone replacement therapy (TRT) in adolescents with KF has been advocated to allow appropriate pubertal development, hormonal stimulation prior to sperm retrieval in KF is not well studied. There is a paucity of studies on the subject and those that have been done are not randomised and thus, conclusions are limited.
We recommend close collaboration with a reproductive endocrinology specialist to hormonally optimise patients prior to surgical sperm retrieval. Patients should not be given testosterone because of the deleterious effective on spermatogenesis, however, in cases of hypogonadism, testosterone production can be stimulated via the use of recombinant LH and FSH administration. If used, testosterone should be stopped at least six months prior to sperm retrieval to allow for recovery [30,31].
Transmission to offspring?
A significant concern regarding fertility preservation among KF patients is the potential transmission of genetic abnormalities to offspring. While the majority of children born to KF parents are reportedly healthy, there are published occasions of foetuses or embryos diagnosed with 47 XXY karyotype [32-34]. Of additional concern is a 2003 study recommending preimplantation screening due to the increase rate of aneuploidy in KF couples [35]. The literature on this topic is still evolving. We highlight that transmission to offspring is theoretically possible and strongly recommend involvement of a genetic counselor.
Looking into the future – spermatogonial stem cell preservation
Based on the idea that germ cell loss starts with the onset of puberty, some have advocated for the preservation of adolescent or even pre-pubertal testicular tissue with the hope of using spermatogonial stem cells to derive sperm in the future. Many centres around the world, including ours, have begun cryopreservation of pre-pubertal testicular tissue in patients prior to the start of chemotherapy but it has not yet become common practice for KF. Deriving sperm from this tissue has been extensively studied in animal models, however this has not been reproduced in humans or non-human primates [36]. Factors to consider are cost, the ethical challenges of subjecting adolescents to an operation they may not understand, and the still unproven benefit of being able to use this technology in humans. At this point, experts in this field believe that this should be reserved for research purposes but not for the regular clinical use [27].
Conclusion
In summary, urology providers are most likely to encounter KF patients in the setting of infertility, sexual dysfunction, or hypogonadism. We recommend a full hormonal evaluation and referral to a centre with expertise in male infertility. Currently, our unit works closely with reproductive endocrinology specialists to hormonally optimise patients and eventually proceed on to mTESE and, if successful, cryopreservation of sperm. Patients should be referred for extensive genetic counseling and the use of preimplantation genetic diagnosis should be considered. There may be a role for preservation of testicular tissue for future use of spermatogonial stem cells however this therapy is still considered experimental.
References
1. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet 2004;364(9430):273-83.
2. Bonomi M, Rochira V, Pasquali D, et al. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest 2017;40(2):123-34.
3. Simpson JL, de la Cruz F, Swerdloff RS, et al. Klinefelter syndrome: expanding the phenotype and identifying new research directions. Genet Med 2003;5(6):460-8.
4. Abdelmoula NB, Amouri A, Portnoi M-F, et al. Cytogenetics and fluorescence in situ hybridization assessment of sex-chromosome mosaicism in Klinefelter’s syndrome. Ann Genet 2004;47(2):163-75.
5. Paduch DA, Fine RG, Bolyakov A, Kiper J. New concepts in Klinefelter syndrome. Curr Opin Urol 2008;18(6):621-7.
6. Greco E, Scarselli F, Minasi MG, et al. Birth of 16 healthy children after ICSI in cases of nonmosaic Klinefelter syndrome. Hum Reprod 2013;28(5):1155-60.
7. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab 2003;88(2):622-6.
8. Aksglaede L, Garn ID, Hollegaard MV, et al. Detection of increased gene copy number in DNA from dried blood spot samples allows efficient screening for Klinefelter syndrome. Acta Paediatr 2012;101(12):e561-3.
9. Forti G, Corona G, Vignozzi L, et al. Klinefelter’s syndrome: a clinical and therapeutical update. Sex Dev 2010;4(4-5):249-58.
10. Jungwirth A, Diemer T, Kopa Z, et al. EAU Guidelines on Male Infertility. 2018.
https://uroweb.org/guideline/
male-infertility/
[Accessed 8 October 2019].
11. Mehta A, Bolyakov A, Roosma J, et al. Successful testicular sperm retrieval in adolescents with Klinefelter syndrome treated with at least 1 year of topical testosterone and aromatase inhibitor. Fertil Steril 2013;100(4):970-4.
12. Samplaski MK, Lo KC, Grober ED, et al. Phenotypic differences in mosaic Klinefelter patients as compared with non-mosaic Klinefelter patients. Fertil Steril 2014;101(4):950-5.
13. Aksglaede L, Wikström AM, Rajpert-De Meyts E, et al. Natural history of seminiferous tubule degeneration in Klinefelter syndrome. Hum Reprod Update 2006;12(1):39‑48.
14. Crüger D, Toft B, Agerholm I, et al. Birth of a healthy girl after ICSI with ejaculated spermatozoa from a man with non-mosaic Klinefelter’s syndrome. Hum Reprod 2001;16(9):1909-11.
15. Bourne H, Stern K, Clarke G, et al. Delivery of normal twins following the intracytoplasmic injection of spermatozoa from a patient with 47,XXY Klinefelter’s syndrome. Hum Reprod 1997;12(11):2447-50.
16. Tournaye H, Staessen C, Liebaers I, et al. Testicular sperm recovery in nine 47,XXY Klinefelter patients. Hum Reprod 1996;11(8):1644-9.
17. Palermo GD, Schlegel PN, Sills ES, et al. Births after intracytoplasmic injection of sperm obtained by testicular extraction from men with nonmosaic Klinefelter’s syndrome. N Engl J Med 1998;338(9):588-90.
18. Ramasamy R, Ricci JA, Palermo GD, et al. Successful Fertility Treatment for Klinefelter’s Syndrome. J Urol 2009;182(3):1108-13.
19. Okada H, Goda K, Muto S, et al. Four pregnancies in nonmosaic Klinefelter’s syndrome using cryopreserved-thawed testicular spermatozoa. Fertil Steril 2005;84(5):1508.
20. Okada H, Goda K, Yamamoto Y, et al. Age as a limiting factor for successful sperm retrieval in patients with nonmosaic Klinefelter’s syndrome. Fertil Steril 2005;84(6):1662-64.
21. Bakircioglu ME, Ulug U, Erden HF, et al. Klinefelter syndrome: does it confer a bad prognosis in treatment of nonobstructive azoospermia? Fertil Steril 2011;95(5):1696-9.
22. Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab 2005;90(11):6263-7.
23. Yarali H, Polat M, Bozdag G, et al. TESE-ICSI in patients with non-mosaic Klinefelter syndrome: a comparative study. Reprod Biomed Online 2009;18(6):756-60.
24. Koga M, Tsujimura A, Takeyama M, et al. Clinical comparison of successful and failed microdissection testicular sperm extraction in patients with nonmosaic Klinefelter syndrome. Urology 2007;70(2):341-5.
25. Schlegel PN. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14(1):131-5.
26. Franik S, Hoeijmakers Y, D’Hauwers K, et al. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Hum Reprod 2016;31(9):1952-9.
27. Gies I, Oates R, De Schepper J, Tournaye H. Testicular biopsy and cryopreservation for fertility preservation of prepubertal boys with Klinefelter syndrome: a pro/con debate. Fertil Steril 2016;105(2):249-55.
28. Corona G, Pizzocaro A, Lanfranco F, et al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: A systematic review and meta-analysis. Hum Reprod Update 2017;23(3):265-75.
29. Fullerton G, Hamilton M, Maheshwari A. Should non-mosaic Klinefelter syndrome men be labelled as infertile in 2009? Hum Reprod 2010;25(3):588-97.
30. Paduch DA, Bolyakov A, Cohen P, Travis A. Reproduction in men with Klinefelter syndrome: the past, the present, and the future. Semin Reprod Med 2009;27(2):137-48.
31. Gravholt CH, Jensen AS, Høst C, Bojesen A. Body composition, metabolic syndrome and type 2 diabetes in Klinefelter syndrome. Acta Paediatr 2011;100(6):871‑7.
32. Reubinoff BE, Abeliovich D, Werner M, et al. A birth in non-mosaic Klinefelter’s syndrome after testicular fine needle aspiration, intracytoplasmic sperm injection and preimplantation genetic diagnosis. Hum Reprod 1998;13(7):1887-92.
33. Ron-El R, Strassburger D, Gelman-Kohan S, et al. A 47,XXY fetus conceived after ICSI of spermatozoa from a patient with non-mosaic Klinefelter’s syndrome: case report. Hum Reprod 2000;15(8):1804-6.
34. Friedler S, Raziel A, Strassburger D, et al. Outcome of ICSI using fresh and cryopreserved-thawed testicular spermatozoa in patients with non-mosaic Klinefelter’s syndrome. Hum Reprod 2001;16(12):2616-20.
35. Staessen C, Tournaye H, Van Assche E, et al. PGD in 47,XXY Klinefelter’s syndrome patients. Hum Reprod Update 2003;9(4):319-30.
36. Fayomi AP, Peters K, Sukhwani M, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019;363(6433):1314-19.
Declaration of competing interests: None declared.




