Introduction
Testicular cancer is the most frequently occurring solid tumour in men between the ages of 15 and 34 years [1]. About 60% of cases are seminomas and approximately 70-80% of them have, at presentation, clinical stage I disease. This article will focus on the current treatment recommendations for stage I seminoma only.
Presentation and diagnosis
Presentation of testicular cancer is usually with a unilateral painless lump in the body of the testis although occasionally pain is present and usually attributed to mild scrotal trauma. Tumour markers for testicular cancer include alpha-fetoprotein (AFP), beta human chorionic gonadotrophin (βHCG) and lactate dehydrogenase (LDH). AFP is NOT secreted by pure seminoma and only 10-20% of patients with pure seminoma have an elevated serum βHCG. If AFP is elevated in a tumour diagnosed as seminoma based on histology, it is reclassified as a non-seminomatous germ cell tumour [2].
Scrotal ultrasound, which has a sensitivity of almost 100% in diagnosing testicular cancer, should be performed to confirm the diagnosis.
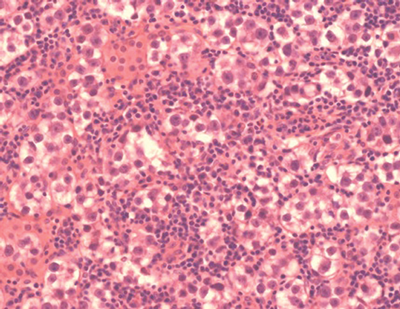
Figure 1: Histopathological image of seminoma showing pleomorphic
malignant cells with large nuclei and a moderate lymphocytic infiltrate.
Surgical treatment of the primary tumour
Radical orchidectomy through an inguinal incision with ligation of the spermatic cord at the level of the internal inguinal ring is the initial standard treatment for seminoma in order to achieve definitive local control [3].
Organ-preserving surgery is an alternative option for patients with synchronous bilateral tumours, metachronous contralateral tumours or lesions in a solitary testis with normal preoperative testosterone levels, if the primary tumour is small. Postoperative local irradiation is recommended in the presence of associated testicular intraepithelial neoplasia but this may be delayed in patients who wish to father children [3].
Staging
Staging is usually undertaken after orchidectomy and helps determine prognosis and treatment. This includes a computed tomography (CT) of the thorax, abdomen and pelvis but additional imaging may be performed depending on the patient symptoms. Clinical stage I seminoma is defined as disease confined to the testicle with no radiological evidence of metastatic disease.
Serum tumour markers should be measured before orchidectomy and five to seven days later. Raised markers should fall after orchidectomy so patients with clinical stage 1 disease where the tumour markers remain elevated despite orchidectomy are presumed to have metastatic disease, even if their CT is normal [2].
Postoperative management
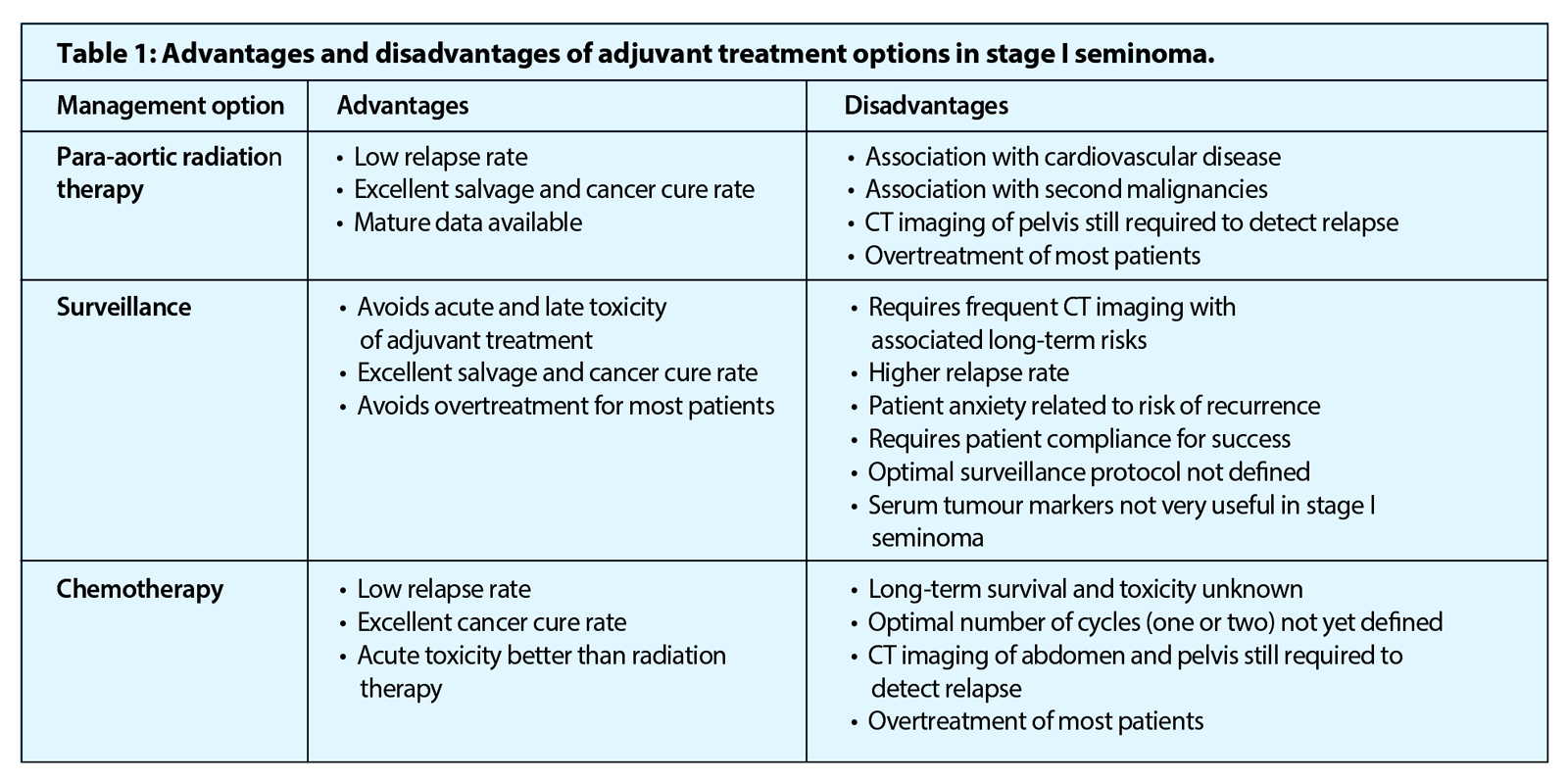
Fifteen to twenty percent of clinical stage I seminoma patients have subclinical metastatic disease and will relapse after orchidectomy alone. The postoperative management has changed over recent years but the optimal strategy remains an area of opinion and debate. Stage I seminoma can be managed by either a surveillance strategy or adjuvant therapy after orchidectomy.
Prognostic risk factors
Two significant predictors for relapse in stage I seminoma were identified retrospectively in a pooled analysis of 638 patients: tumour size >4cm and invasion of the rete testes. Patients with two adverse prognostic factors had a five-year relapse rate of 31.5%, compared with 12.2% in patients without any [4]. This observation has not been validated in a prospective setting, except in that the absence of both factors indicated a low recurrence rate (6%) [5].
The results of a large Danish surveillance study, which included 1822 patients with stage I seminoma who were managed with surveillance, presented by Mette Sakso Mortensen at the American Society of Clinical Oncology (ASCO) annual meeting in 2013, identified invasion of blood or lymphatic vessels, tumour size >4cm and serum HCG >200IU/L as significant prognostic risk factors for relapse [6].
Radiotherapy
Seminomas are radiosensitive tumours and for several decades, post orchidectomy irradiation of the para-aortic +/- ipsilateral pelvic lymph nodes was the standard treatment in stage I seminoma. Adjuvant radiotherapy has a relapse rate of <5% but has become less favoured and is no longer recommended by the European Association of Urology [7] after several studies confirmed its association with increased risk of cardiovascular toxicity and a second malignancy [8].
Modern radiotherapy, however, involves smaller fields and lower doses than those used in the past and the National Comprehensive Cancer Network guidelines recommend radiotherapy as an option for treatment in patients where surveillance is not applicable. They recommend a total dose of 20Gy in 10 fractions, given to the infradiaphragmatic area [1].
“Surveillance avoids both the acute and long-term toxicity associated with adjuvant treatments.”
Surveillance
The concerns surrounding the long-term effects of radiation therapy and the success of surveillance in stage I non-seminomatous germ cell tumours led to the exploration of this strategy as a management option in stage I seminoma.
Surveillance involves routine CTs of the abdomen along with physical examination, measurement of serum βHCG and chest radiographs to detect relapse, with the initiation of treatment should relapse occur. Almost all relapses can be rescued successfully with platinum-based chemotherapy. The optimal follow-up schedule and frequency of investigations, however, remains unclear. A clinical trial in the UK which aims to enrol 660 patients, entitled TRISST (Trial of Imaging and Schedule in Seminoma Testis) is currently studying whether magnetic resonance imaging (MRI) scans are as effective as CT scans in detecting relapse and the optimum number of scans for this type of monitoring [9]. Patients are randomised to one of four surveillance arms. In arm I, patients undergo CT of the abdomen / retroperitoneum at 6, 12, 18, 24, 36, 48, and 60 months, in the absence of disease progression, and at 6, 18, and 36 months in arm II. In arm III, patients undergo MRI of the abdomen / retroperitoneum at 6, 12, 18, 24, 36, 48, and 60 months, and at 6, 18 and 36 months in arm IV.
Surveillance avoids both the acute and long-term toxicity associated with adjuvant treatments and has been accepted as a safe and effective management option over the last 25 years. The relapse rate is, however, in the order of 15-20% at five years, which is higher when compared to adjuvant radiotherapy or chemotherapy [5]. Although the relapse rate is higher, there is no reduction in the cancer-specific survival rate, which remains 97-100% [5,10].
The main disadvantage of surveillance is that compliance is a key factor for its success. It requires commitment to reasonably intense follow-up from both patients and clinicians. The median time to relapse post-orchidectomy ranges from 12-18 months but there is a clinically significant risk of relapse more than five years after orchidectomy, which supports the need for prolonged follow-up [11].
Chemotherapy
Adjuvant chemotherapy in the form of single drug, carboplatin, is the latest addition to the available treatment options for stage I seminoma, which was explored after concerns regarding the long-term complications of radiotherapy and higher relapse rates with surveillance.
A Medical Research Council (MRC) randomised trial of 1447 patients, comparing one cycle of carboplatin (at seven x AUC dose) with radiotherapy as adjuvant treatment for stage I seminoma, found carboplatin had a non-inferior relapse free rate of 94.7% at five years compared to 96% for radiotherapy (per protocol analysis: HR, 1.27; 90% CI, 0.83 to 1.92; P=0.36) and a statistically significant reduction in contralateral germ cell tumours (carboplatin, n=2; RT, n=15; HR, 0.22; 95% CI, 0.05 to 0.95; P=0.03) [12]. Compared with radiotherapy, carboplatin was associated with significantly more thrombocytopenia, but significantly less dyspepsia and lethargy, and a more rapid return to work [13]. There is insufficient long-term data to comment definitively on any late side-effects associated with single agent carboplatin and since most of the relapses occurred in the retroperitoneal / para-aortic lymph nodes, CT monitoring for relapse is still required after treatment. However, despite this, adjuvant carboplatin is the preferred treatment option in the UK and Europe for those patients deemed unsuitable for surveillance.
Conclusion
Five-year overall survival for stage I seminoma is >95% regardless of the postoperative management [8] but adjuvant treatment exposes the 80% of patients who would never relapse after orchidectomy to the risk of treatment-related toxicity. Surveillance minimises the toxicity associated with adjuvant treatment, while preserving high cure rates and has become a well-established treatment option worldwide. One third of panellists at the Third European Consensus Conference on Diagnosis and Treatment of Germ-Cell Cancer considered surveillance the preferred treatment strategy in all patients regardless of the risk factors for relapse [14]. Others opted for surveillance in ‘low risk’ patients and adjuvant treatment in ‘high risk’ patients but this can only be achieved by identifying reliable prognostic markers.
The management of stage I seminoma remains controversial. The available evidence should be presented to patients and their preferences must be considered in order to select the most appropriate option for the individual.
References
1. Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: testicular cancer. J Natl Compr Canc Netw 2009;7(6):672-93.
2. Ehrlich Y, Beck SD, Foster RS, et al. Serum tumor markers in testicular cancer. Urol Oncol 2013;31(1):17-23.
3. Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): part I. Eur Urol 2008;53(3):478-96.
4. Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol 2002;20(22):4448-52.
5. Aparicio J, Germà JR, García del Muro X, et al. Risk-adapted management for patients with clinical stage I seminoma: the Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol 2005;23(34):8717-23.
6. American Society of Clinical Oncology (ASCO) – Daily News – Post-Surgery Surveillance Adequate for Patients with Stage I Seminoma. 2013.
http://am.asco.org/post-surgery-
surveillance-adequate-patients-
stage-i-seminoma
Last accessed June 2014.
7. Albers P, Albrecht W, Algaba F, et al. EAU guidelines on testicular cancer: 2011 update. Eur Urol 2011;60(2):304-19.
8. Chung P, Mayhew LA, Warde P, et al. Management of stage I seminomatous testicular cancer: a systematic review. Clin Oncol (R Coll Radiol) 2010;22(1):6-16.
9. MRC Clinical Trials Unit – Study Details – TRISST (MRC TE24): Trial of imaging and schedule in seminoma testis 2014.
http://www.ctu.mrc.ac.uk/
research_areas/study_
details.aspx?s=40
Last accessed June 2014.
10. Tandstad T, Smaaland R, Solberg A, et al. Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish norwegian testicular cancer study group. J Clin Oncol 2011;29(6):719-25.
11. Chung P, Parker C, Panzarella T, et al. Surveillance in stage I testicular seminoma – risk of late relapse. Can J Urol 2002;9(5):1637-40.
12. Oliver RT, Mead GM, Rustin GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol 2011;29(8):957-62.
13. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet 2005; :293-300.
14. Beyer J, Albers P, Altena R, et al. Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol 2013;24(4):878-88.