Case 1
- A 32-year-old female patient is diagnosed with a ureteric calculus for the first-time. What type of metabolic evaluation investigations should be performed?
- When should stone analysis be repeated?
- What are the most common metabolic abnormalities associated with calcium oxalate stones?
- What is the role of citrate in urine?
- The patient’s urinary calcium excretion is noted to be >8mmol/day. What pharmacological treatment may be appropriate?

Figure 1
Case 2
- What are these stones?
- What appearance would the crystals have on light microscopy?
- What would be the typical density of this stone in HU?
- What pharmacological treatment options are available for this stone type?
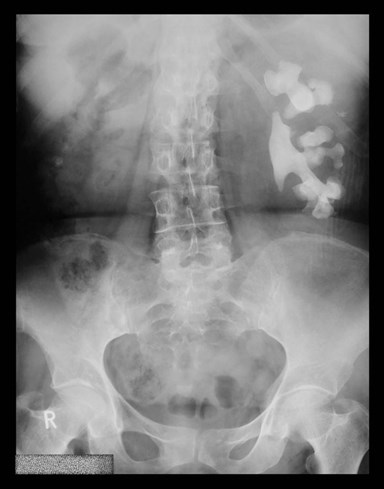
Figure 2
Case 3
1. What is the likely composition of this calculus?
2. What is the likely urine pH?
3. What organisms is this stone commonly associated with?
4. Describe the chemical reaction catalysed by urease.cal treatment?
Acknowledgement
Figure 1 reprinted from Daudon M, Jungers P, Bazin D. Stone Morphology: Implication for Pathogenesis. AIP Conference Proceedings 2008;1049:199-215. 10.1063/1.2998023. With the permission of AIP Publishing.
Figure 2 courtesy of Dr Nikos Karapasias, Radiopaedia.org, rID: 25349
https://radiopaedia.org/cases/staghorn-calculi-coral-calculi?lang=gb
Urolithiasis – metabolic considerations: answers
Case 1
1. Basic metabolic evaluation:
- Blood tests (urea and electrolytes (U&Es), serum urate / calcium). Parathyroid hormone (PTH) test if raised serum calcium.
- Urinalysis including pH (high in infection, low in uric acid), urine culture.
- Stone analysis (infrared spectroscopy or X-ray diffraction) should be performed in all first-time stone patients [1].
Additional tests if deemed ‘high-risk’ stone former:
- Two consecutive 24-hour urine collection (calcium, oxalate and citrate require collection in an acid as they may precipitate by complexing with ions during collection) analysed at minimum for total volume, pH, calcium, oxalate, uric acid, citrate, sodium, potassium, creatinine (~0.2mmol/kg/day, represents quality of urine collection).
- 24-hour cystine if suspected.
Factors for higher risk of recurrent stone formation:
- Early onset of urolithiasis (especially children / teenagers).
- Recurrent stones / short time since last stone episode.
- Residual stone fragments.
- Family history.
- Stone type (uric acid, cystine, struvite, brushite).
- Nephrocalcinosis.
- Comorbidities (gout, recurrent urinary traction infections, bowel malabsorption, hyperparathyroidism, osteoporosis).
- Anatomical abnormalities (see European Association of Urology Urolithiasis Guideline for comprehensive list [1]).
2. Repeat stone analysis is needed in cases of:
- Recurrent stones despite pharmacological prevention.
- Early recurrence after complete stone clearance.
- Late recurrence after a prolonged stone-free period.
3. Most patients with calcium stones have one or more metabolic abnormalities. Hypercalciuria affects 30-60% of adult stone formers and hyperoxaluria is found in 26-67%. These are followed by hyperuricosuria (15-46%), hypomagnesuria (7-23%) and hypocitraturia (5-29%). In most cases, the cause of the abnormality itself is unknown but low urine volume is the most important predisposing factor to stone formation.
4. Citrate forms a soluble complex with calcium, preventing calcium combining with oxalate or phosphate to agglomerate into crystals. The primary determinant of urinary citrate excretion is the acid-base balance status – metabolic acidosis reduces citrate excretion as it is metabolised to bicarbonate; metabolic alkalosis (or oral bicarbonate supplementation) increases excretion.
5. Thiazide diuretic to increase calcium resorption. Patient likely has ‘renal leak’ hypercalciuria with impairment of renal tubular resorption of calcium (can be associated with secondary hyperparathyroidism). Other possible sources of hypercalciuria are intestinal absorption (as seen in ‘absorptive hypercalciuria’) and calcium resorption from de-mineralisation of bone.
Case 2
1. Uric acid stone (stone analysis is not always possible so it is useful to be familiar with what different stone types look like!).
2. Rectangular.
3. Uric acid stones have low density (200-450 HU).
4. The majority of uric acid stones (but not sodium or ammonium urate) can be dissolved by oral chemolysis. Information on the stone type can be inferred from prior stone analysis, urinary pH measurements and X-ray characteristics.
Oral chemolysis works by raising the pH of urine by using alkaline citrate or sodium bicarbonate (potassium citrate is preferred as sodium may inhibit calcium resorption leading to hypercalciuria and thus calcium oxalate stone formation). Urinary pH should be adjusted to 7.0-7.2 (chemolysis is more effective at a higher pH but this may promote calcium phosphate stone formation). In addition to careful patient follow-up during oral chemolysis, EAU guidelines recommend that patients also monitor their urine pH by dipstick and modify dosage of alkalising medication accordingly as there is direct dose correlation to pH.
Case 3
1. Struvite (magnesium ammonium phosphate) or ‘triple phosphate’ accounts for 70% of Staghorn calculus and usually mixed with calcium phosphate rendering them radio-opaque on plain KUB or CT KUB.
2. Struvite only precipitates at pH >7.2.
3. For struvite stones to develop, it requires an alkaline pH and ammonium in urine which is driven by urease-producing organisms (e.g. Proteus, Morganella, Klebsiella, Enterobacter). Urease from bacteria hydrolyses urea to ammonia and CO2.
4. Urease enzymes catalyse the hydrolysis of urea: H2O + CH4N2O (urea) n 2NH3 (ammonia) + CO2. This in turn leads to ammonium production: 2NH3 (ammonia) + H2O g 2NH4+ (ammonium) + 2OH-.
References
1. European Association of Urology. Urolithiasis Guideline. EAU Guidelines Office; Arnhem, the Netherlands; 2021.
2. Reynard J, Brewster S, Biers S & Neal N. Oxford Handbook of Urology, 4th edition, Oxford University Press, 2019.
3. Ratkalkar, Vishal N, and Jack G Kleinman. “Mechanisms of Stone Formation.” Clinical reviews in bone and mineral metabolism vol. 9, 3-4 (2011): 187-197. doi:10.1007/s12018-011-9104-8.
4. Johnston T, Rochester M & Wiseman O. “Urinary Tract Stones.” Viva Practice for the FRCS(Urol) and Postgraduate Urology Examinations, edited by Arya M et al., CRC Press, 2018, 219-242.







