In January 2016, the UK National Screening Committee once again recommended against a systematic population screening programme for prostate cancer due to the, as yet, insufficient evidence that the benefits of screening would outweigh the harm to the population as a whole [1].
The recently updated Prostate Cancer Risk Management programme (PCRMP) from Public Health England [2] supports this view, as do many professional associations, including the British Association of Urological Nurses (BAUN), as well as groups representing patients, such as Prostate Cancer UK.
The PCRMP states that any asymptomatic man aged 50 and over can make an appointment with their GP to discuss having the prostate specific antigen (PSA) test but that GPs should not proactively raise the issue with these men. This guidance is based on good-quality evidence-based information. However, despite guidance and opinion from many sources regarding the use of PSA testing in the detection of prostate cancer [3-10], there remain areas of use for PSA (as a diagnostic tool) where robust evidence does not exist. More clarity would reduce some of the uncertainty in how to offer balanced, evidence-based, yet pragmatic information to asymptomatic men concerned about prostate cancer who request PSA testing.
Current guidance relies on the asymptomatic man being proactive with regards to his health, being aware of PSA and its implications, and initiating a consultation to discuss obtaining a test. This limits the benefits of PSA testing (and indeed the risks) to the demographic of men who display greater proactivity towards their health. In addition (due to lack of level 1 evidence) it does not take into account the men under the age of 50 who are at increased risk of prostate cancer, those who would benefit from a baseline PSA test in their 40s to help predict future risk, and those who would not gain any benefit from prostate cancer detection due to their limited life expectancy.
In recognising these gaps and to provide support to primary healthcare professionals (HCPs) in these areas, in the absence of level 1 evidence, BAUN, along with a number of other professional bodies, have been involved in the development of a clinical consensus to cover these additional areas. A process which was led by Prostate Cancer UK.
The consensus statements [11] were developed using the Delphi survey technique, a consensus method commonly used within health and social sciences to enhance effective decision-making in areas where limited research or a lack of clarity exist. Three hundred and thirty-five HCPs took part. A steering group of experts, with an independent Chair, was then convened to agree the final wording of the statements. I represented BAUN on this steering group.
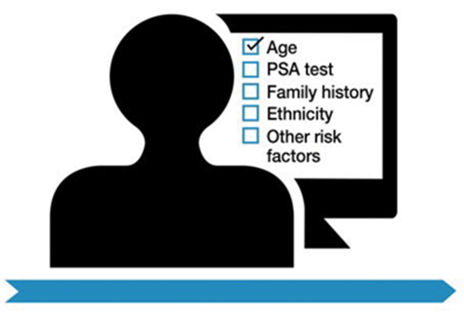
Figure 1 (Courtesy of Prostate Cancer UK).
The set of 13 statements recommend that Governments and public health agencies have primary responsibility for raising awareness of prostate health and prostate cancer risk factors amongst men in the UK, with relevant contributions from HCPs and charities, and that the use of targeted messaging should be considered. This included the following:
- Few men in the UK have a ‘baseline’ PSA test but the new consensus highlights to all HCPs that baseline testing should be an NHS option for all men over the age of 40 across the UK who request it, in order to predict their future risk of developing prostate cancer. If the PSA level is above the age-specific median value, they should be considered at higher than average risk of prostate cancer and should be encouraged to be re-tested in the future. The age-specific median value for men aged 40-49 years is 0.7ng/ml.
- The statements recommend that primary HCPs should discuss the PSA test with asymptomatic men who are at higher than average risk of the disease (including Black ethnicity and family history of prostate cancer) and that this should occur from the age of 45 (Figure 1).
- PSA history and increasing PSA (even whilst still under the referral threshold) should be taken into consideration when deciding whether to refer to secondary care. The PCRMP states the new recommended prostate biopsy referral value for men aged 50-69 years is ≥3ng/ml.
- Asymptomatic men with a life expectancy clearly less than 10 years should be advised not to have a PSA test due to the lack of benefit. The statement acknowledges that further work is required to better estimate an individual’s life expectancy.
These UK PSA consensus statements provide clarity to the aspects of PSA testing in asymptomatic men where level 1 evidence is not available. They provide more information for primary HCPs and should be used to supplement Public Health England’s guidance, though not to replace it. A plain English version has also been developed to help provide additional information for the broader public to raise awareness of risk factors and the PSA test [12].
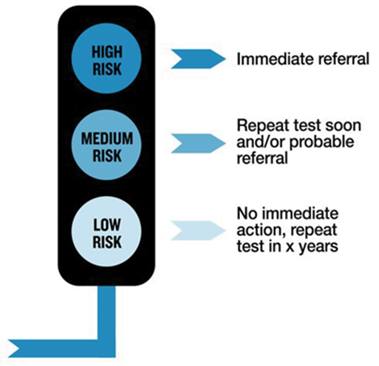
Figure 2 (Courtesy of Prostate Cancer UK).
Looking to the longer term, Prostate Cancer UK recently announced a new project to develop a new risk assessment tool for primary care to give a better indication of a man’s risk of getting prostate cancer, and how agressive it is likely to be [13] (Figure 2). Part of its development will also include a better predicitve method of life expectancy. It is hoped it will be in the hands of all GPs within five years.
As with the PCRMP, the consensus statement recommendations do not recommend a population-wide screening programme. They focus on future risk of developing prostate cancer using what is known about PSA levels in men under 50 (before the PSA may rise to exceed the 3ng/ml referral level), PSA velocity, those with a less than 10-year life expectancy, and in those with an increased risk of developing prostate cancer.
“It is vital that all men have access to accurate and balanced information regarding the risks and benefits of PSA testing for their own individual circumstances.”
It is vital that all men have access to accurate and balanced information regarding the risks and benefits of PSA testing for their own individual circumstances, to understand their individual risk and be guided through the process of deciding whether PSA testing is right for them. The consensus statements set this out clearly and will be a valuable aid to all those involved in the decision-making process.
Nurses are ideally placed to have such discussions; however, specialist urology nurses remain largely based in secondary care with only 7% being based in the community [14]. The need for knowledgeable primary care based practitioners able to proactively discuss the risks and benefits of PSA testing in asymptomatic men is highlighted by these consensus statements which demonstrate the complexity of the discussion required as part of decision-making.
Further Information
Visit www.prostatecanceruk.org/PSAconsensusHP for further information.
References
1. UK National Screening Committee. The UK NSC recommendation on Prostate cancer screening/PSA testing in men over the age of 50. 2016. Available from:
http://legacy.screening.nhs.uk/prostatecancer
2. Public Health England. Prostate cancer risk management programme: overview. 2016. Available from:
https://www.gov.uk/guidance/prostate-cancer-
risk-management-programme-overview
3. Bell N, Gorber SC, Shane A, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. Can Med Assoc J 201;186(16):1225-34.
4. Prostate Cancer Foundation of Australia; Cancer Council Australia. Clinical practice guidelines PSA Testing and Early Management of Test-Detected Prostate Cancer - Cancer Guidelines Wiki. Available from:
http://wiki.cancer.org.au/australia/Guidelines:PSATesting
5. Murphy DG, Ahlering T, Catalona WJ, et al. The Melbourne Consensus Statement on the early detection of prostate cancer. BJU Int 2014;113(2):186-8.
6. Public Health England. Prostate cancer risk management programme (PCRMP): benefits and risks of PSA testing. 2016. Available from:
https://www.gov.uk/government/publications/
prostate-cancer-risk-management-programme-
psa-test-benefits-and-risks/prostate-cancer-
risk-management-programme-pcrmp-
benefits-and-risks-of-psa-testing
7. Moyer VA; U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157(2):120-34.
8. Heidenreich A, Abrahamsson P-A, Artibani W, et al. Early detection of prostate cancer: European association of urology recommendation. Eur Urol 2013;64(3):347-54.
9. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA Guideline. J Urol 2013;190(2):419-26.
10. National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology (NCCN Guidelines). Prostate Cancer Early Detection v1.2014. 2012. Available from:
http://www.nccn.org
11. Prostate Cancer UK. Consensus statements on PSA testing in asymptomatic men in the UK (Health Professionals version). 2016. Available from:
www.prostatecanceruk.org/PSAconsensusHP
12. Prostate Cancer UK. Consensus statements on PSA testing in asymptomatic men in the UK (Plain English version). 2016. Available from:
www.prostatecanceruk.org/PSAconsensus
13. Prostate Cancer UK. New risk tool for GPs set to revolutionise prostate cancer diagnosis within five years. Available from:
http://prostatecanceruk.org/about-us/
news-and-views/2016/2/new-risk-tool-for-gps-
set-to-revolutionise-prostate-cancer-
diagnosis-within-five-years
14. Leary A, Brocksom J, Endacott R, et al. The specialist nursing workforce caring for men with prostate cancer in the UK. International Journal of Urological Nursing 2016;10:5-13.
Declaration of Competing Interests: None declared.




