Introduction
The ejaculatory process is paramount to procreation in nature. It is a complex orchestration of physiology that results in emission of the ejaculate into the posterior urethra followed by ejection of those fluids from the urethra and orgasm. The study of ejaculation is a recent innovation within the field of medicine, with research in the last few decades revealing the true complexity of both normal and abnormal pathways.
Public and professional awareness of male sexual dysfunction has increased in part due to the rise in phosphodiesterase inhibitor use for erectile dysfunction. The old belief of sexual dysfunction lying purely within the domain of the aged man has been shattered. Lauman’s study of 1410 men aged 18-59 years revealed sexual dysfunction in up to 31% [1]. More recent studies show similar results with estimates of sexual dysfunction in up to 28% [2].
Sexual dysfunction encompasses hypogonadism (primary and secondary), erectile dysfunction and ejaculatory dysfunction. The most common of these is ejaculatory dysfunction, which can affect up to 14% of men [2].
Ejaculatory dysfunction can manifest as a spectrum that ranges from the most common, premature ejaculation (PE), through to the lesser seen delayed and absent ejaculation (anejaculation). In retrograde ejaculation the ejaculate does not enter the posterior urethra but retro pulses into the bladder only to exit the urethra with the next urinary void. Whereas PE is largely thought to arise from psychological pathology, AE and RE are mainly due to an organic cause.
Normal ejaculatory physiology
The normal ejaculatory process is a complex interplay between sensory receptors, afferent pathways, cerebral sensory and motor centres, spinal motor centres and efferent pathways (Figure 1). This multifaceted interplay results in emission and expulsion with orgasm.
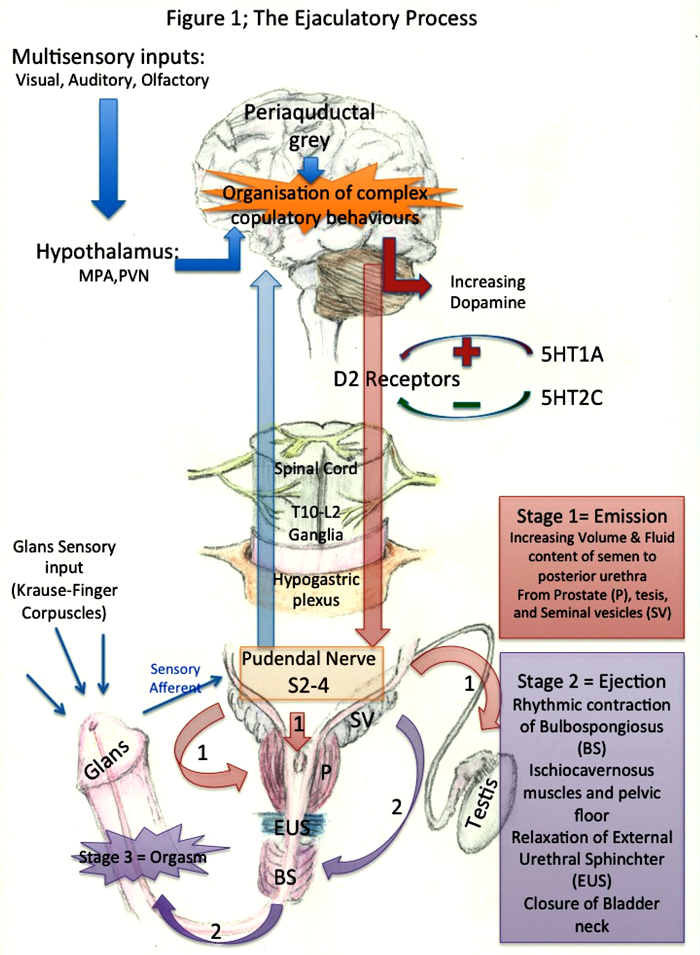
Figure 1: the ejaculatory process.
Initial stimulation of the Krause-Finger corpuscles in the glans mucosa of the penis results in reflex activation once a threshold has been achieved. This sensory information is transmitted via the dorsal nerve of the penis (S4) to the lumbosacral spinal cord combining with sympathetic afferents from the hypogastric plexus. This combined input ascends the cord and receives further visual, auditory and olfactory input in the cerebrum.
This multi-sensory input then has to be co-ordinated by the forebrain to form the complex of copulatory behaviours. These forebrain structures include the medial pre-optic area (MPA) and the para-ventricular nucleus (PVN) of the hypothalamus.
There are several neurotransmitters involved in the central ejaculatory pathway and in the last decade their role has become clearer. Dopamine levels increase during sexual excitation and this in turn has an excitatory role on ejaculation (via D2 receptors) [3]. GABA-receptor antagonists have demonstrated sexual inhibition in animal models. Oxytocin mediates the muscular contraction involved in ejaculation. The double-edged sword of serotonin (5-Hydroxytryptamine-HT) has both an inhibitory role via 5-HT2C receptors and an excitatory role via 5-HT1A receptors [4,5]. Nitric oxides modulates seminal emission [6].
The sympathetic efferents (T10-L2) conduct the emission aspect of the reflex. These activated fibres curve over the pelvic brim to the pelvic plexus and result in sequential contraction of the epididymis, vas deferens, seminal vesicle and prostate with co-ordinated closure of the bladder neck.
The somatically driven pudendal nerve initiates the expulsion or ejection phase (S2-S4) resulting in rhythmic contractions of the bulbospongiosus and bulbocavernosus muscles and of the pelvic floor expelling the semen with enough force to exit the urethra. The external urethral sphincter relaxes and the bladder neck closes resulting in antegrade ejaculation.
The pressure increase in the posterior urethra is relayed through the sensory pudendal afferents in association with sensory stimuli from the verumontanum, urethral contraction and input from secondary sexual organs. After central processing this results in orgasm.
Premature ejaculation
This was first described by Shapiro in 1947 and was divided into two types which were later termed lifelong and acquired. The prevalence of lifelong or chronic PE is about 30% [1]. Acquired PE may come on gradually or suddenly but is preceded by a normal intravaginal ejaculatory latency time (IELT).
The International Society for Sexual Medicine definition (2007) for PE is IELT of less than one minute for lifelong PE. For acquired PE, where the man had previously experienced normal intravaginal latency times the definition has been recently defined as a clinically significant and bothersome reduction in latency time, often to about three minutes [7]. This involves negative personal consequences such as distress, anxiety and bother. In severe cases it may lead to avoidance of intercourse completely.
The prevalence of PE can be as high as 30% from the global study of sexual attitudes and behaviours in men over 40 [2]. Although, due to relative inconsistency of symptoms and degree of bother, the true prevalence is difficult to measure.
Causes of PE
For lifelong PE Waldinger proposed central serotinergic dysfunction as the causative agent, being either a hyposensitivity of the 5-HT2C and or hypersensitivity of the 5-HT1A receptor and not a psychological problem [8].
In acquired PE the causes can be psychosexual or relationship-based issues but they may also be organic in nature too, such as erectile dysfunction, prostatitis or thyroid dysfunction.
Non-pharmacological treatment of premature ejaculation
The treatment of PE is dependent on the type – lifelong or acquired – and the likely cause. Urological issues and thyroid problems need to be managed appropriately. If there is a psychological element then it too needs addressing.
Men with PE are often racked with feelings of guilt and failure, which in turn leads to worry and tension. Relative to men without PE they also have decreased self-confidence and are overly occupied with their ejaculation. This can often lead to relationship dysfunction [9].
Present day psychotherapy embodies the concepts of behavioural techniques such as stop-start and pause-squeeze methods [10] with cognitive approaches and short-term psychotherapy. Current thinking supports lifelong PE ejaculation as a neurobiological phenomenon based on animal models and not a psychological problem [8].
This strategy can be expensive and requires a specialty service to be set up for men to be referred. The benefits are plentiful, in that the techniques learned have no side-effects, have no impact on men’s other comorbidities and are tailored to the individual’s situation. This also allows the man to communicate his sexuality with his partner and increase bonding. The techniques can be used again for current and future relationships as necessary.
Pharmacotherapy for premature ejaculation
Topical anaesthesia can be used directly on to the glans penis, however this must be worn with a condom lest vaginal anaesthesia occur. Studies using TEMPE® (eutectic mixture of prilocaine and lidocaine, Plethora UK), showed an IELT 2.4 times higher than control with improvement in patient and partner sexual quality of life [11].
In recent years selective serotonin reuptake inhibitors (SSRI) have been demonstrated to block axonal re-uptake of serotonin from the synaptic cleft of central and peripheral serotonergic neurones by 5HT transporters. The stimulation of post-synaptic 5HT2c autoreceptors, [12] in respect of paroxetine, sertraline, fluoxetine citalopram and fluvoxamine has also been researched. Paroxetine shows the greatest ejaculation delay (8.8 fold increase over placebo) [13]. Men treated with SSRIs should be counselled as to the risk of adverse events and withdrawal. Currently daily SSRIs are off licence for use in PE.
One of the newer shorter acting SSRIs, dapoxetine, has been shown to have a lower incidence of adverse events and sexual dysfunction. Dapoxetine as an on demand treatment was shown to have significantly improved IELT and patient and partner sexual satisfaction [14]. Dapoxetine is the only SSRI licensed for the treatment of PE.
Phosphodiesterase inhibitors are well known for their role in treating erectile dysfunction and now also in male lower urinary tract obstruction. Initial reports of their use in the treatment of PE were not encouraging [15] but more recent studies have shown them to have a favourable effect in increasing the IELT compared to placebo [16].
The use of both an SSRI and a phosphodiesterase inhibitor (PDE5) has been explored but remains a contraindication because of the PDE5 interaction with CYP2D6 inhibitors (with a likely increase in adverse events as a result). The combination of dapoxetine with sildenafil has shown an increased IELT over dapoxetine alone [17]. Likewise, an increase in IELT has been shown for paroxetine and tadalafil [18]. In these studies men had PE only (and without ED). The increased IELT with dapoxetine has been shown in men with ED and PE on a stable dose of a PDE5 inhibitor with similar adverse events to those on a PDE5 inhibitor alone [19].This would suggest that PDE5 inhibitors have a potential use in PE in men with and without ED and that this is more effective in combination with an SSRI such as dapoxetine. However at present, combination treatment with depoxetine and a PDE5i is still contraindicated under the UK licence.
“Ejaculatory dysfunction is both a common and debilitating problem for men of all ages.”
Other pharmacological approaches which have failed to show significant results for PE include tramadol, A1 adrenoreceptor antagonists and penile injection therapy.
Upon seeing a man with PE in the clinic the urologist should obtain detailed medical and sexual history, physical examination and appropriate investigations i.e. thyroid function tests, urine culture and sensitivity. If an obvious organic cause of PE is found then treatment is tailored accordingly. The patient will need to give an account of the duration of the PE (acute or lifelong, situation or global). A subjective account of the IELT is taken from the patient and an assessment of the partner’s satisfaction made.
Men with lifelong PE are usually best managed pharmacologically. In these instances a decision is made between the more potent daily SSRI, paroxetine (10mg to 40mg daily) which is off-label and the on demand preparation dapoxetine (30mg to 40mg one to two hours before intercourse) which has less side-effects and which has a lower risk of withdrawal (and is also the only licensed SSRI). Paroxetine, although more effective, will take on average five to seven days to increase IELT and can in some cases take up to two to three weeks.
Cognitive behavioural therapy (CBT) is likely to benefit any men with significant relationship factors that are contributing to the PE. Acquired PE can be managed with pharmacotherapy (dapoxetine) [20] with added CBT for the couple.
Retrograde ejaculation
Men who fail to ejaculate but attain orgasm may have a failure of ejaculation or retrograde ejaculation (RE). In RE the sperm is retropulsed into the bladder through an open bladder neck due to the failure of the bladder neck closure. The bladder neck is contracted by sympathetic activation of the α-adrenoreceptors. This can occur during pharmacotherapy for bladder outflow obstruction particularly with the uroselective α-blocker tamsulosin in up to 30% [21]. It is less of an issue with other alpha blockers.
Bladder neck incision and transurethral resection of prostate will surgically disrupt the bladder neck muscle fibres preventing them from closing during ejaculation. The rate of RE after this type of surgery is up to 80% [22]. This point needs to be very clearly explained during the consenting process particularly in men requiring surgery before completing their family. Newer techniques with laser prostate resection may have less RE [23] and certainly new technologies such as the Urolift™ have been developed to preserve bladder neck closure [24].
Other surgical insults resulting in RE can occur during pelvic surgery where the hypogastric plexus crosses the pelvic brim and is susceptible to injury. This is relevant in retroperitoneal lymph node dissection for germ cell tumours, aorto-iliac surgery, and excision of the rectum. These sympathetic fibres can also be damaged as part of diabetes mellitus, multiple sclerosis and spinal cord injury [25].
The diagnosis of RE can be confirmed by testing the post ejaculation void for fructose and spermatozoa and transrectal ultrasound (TRUS) visualisation of an open bladder neck at rest.
Treatment of retrograde ejaculation
The management of RE may, in some cases, only require reassurance to the man that there is no serious underlying pathology. Alpha-blockers can be withdrawn or changed. An attempt to close the bladder neck via α-adrenergic activation with pseudoephidrine or using tricyclic antidepressants such as imipramine with noradrenaline reuptake inhibitors action could be made. If the RE is inhibiting fertility, semen can be extracted from the post orgasmic voided urine. Augmenting the bladder neck with bulking agents such as macroplastique has limited success.
Anejaculation
In extreme cases there may be complete failure of the muscles involved in emission resulting in anejaculation. This is more often seen in spinal cord injury patients [26] but can also been seen after radical surgery of the pelvis. Fertility may be restored with the use of a penile vibrator / electro ejaculator [27]. In those not responding to penile vibratory techniques or in non spinal cord injury anejaculation men, then surgical sperm retrieval and intracytoplasmic sperm insemination are suitable alternatives.
Painful ejaculation
This is an uncommon problem and can also have a psychological or organic cause. Acute and chronic prostatitis can lead to painful ejaculation. Treatment of the prostatitis or other underlying cause of the pain is required. Other pathology that can cause painful ejaculation includes ejaculatory duct inflammation obstruction, inflammation or stones in the ejaculatory duct. The latter two are amenable to endoscopic resection.
Conclusion
Ejaculatory dysfunction is both a common and debilitating problem for men of all ages. A simple approach to define the type of ejaculation as premature, retrograde or anejaculation from the history establishes the likely cause. Medical conditions will need to be optimised if implicated and causative medication can be reviewed. Cognitive behavioural therapy for PE should be used where appropriate. The medical treatment of PE should consider the use of topical therapy with local anaesthetic agents and SSRIs either daily (but this is an off license treatment) or on demand.
In retrograde ejaculation and anejaculation the restoration of normal antegrade flow is unlikely to be achieved. If surgery is to be undertaken with a high risk of RE in men who have not completed their families then this should be borne in mind. Either a limited bladder neck incision, or use of Urolift™, to limit RE should be considered. Alternatively a uro-selective α-blocker other then tamsulosin can be used and surgery deferred until the man has completed his family.
References
1. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281(6):537-44.
2. Nicolosi A, Laumann EO, Glasser DB, et al. Sexual behavior and sexual dysfunctions after age 40: the global study of sexual attitudes and behaviors. Urology 2004;64(5):991-7.
3. Pehek EA, Thompson JT, Hull EM. The effects of intracranial administration of the dopamine agonist apomorphine on penile reflexes and seminal emission in the rat. Brain Research 1989;500(1-2):325-32.
4. Ahlenius S, Larsson K. Evidence for an involvement of 5-HT1B receptors in the inhibition of male rat ejaculatory behavior produced by 5-HTP. Psychopharmacology 1998;137(4):374-82.
5. Lorrain DS, Matuszewich L, Friedman RD, Hull EM. Extracellular serotonin in the lateral hypothalamic area is increased during the postejaculatory interval and impairs copulation in male rats. The Journal of Neuroscience 1997;17(23):9361-6.
6. Tome AR, da Silva JC, Souza AA, et al. Possible involvement of nitric oxide in pilocarpine induced seminal emission in rats. General Pharmacology 1999;33(6):479-85.
7. Althof SE, McMahon CG, Waldinger MD, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE). The Journal of Sexual Medicine 2014;11(6):1392-422.
8. Waldinger MD. The neurobiological approach to premature ejaculation. The Journal of Urology 2002;168(6):2359-67.
9. Hartmann U, Schedlowski M, Kruger TH. Cognitive and partner-related factors in rapid ejaculation: differences between dysfunctional and functional men. World Journal of Urology 2005;23(2):93-101.
10. Masters WH, Johnson V. Human Sexual Inadequacy. Littlebrown; 1970.
11. Dinsmore WW, Hackett G, Goldmeier D, et al. Topical eutectic mixture for premature ejaculation (TEMPE): a novel aerosol-delivery form of lidocaine-prilocaine for treating premature ejaculation. BJU International 2007;99(2):369-75.
12. Rowland D, McMahon CG, Abdo C, et al. Disorders of orgasm and ejaculation in men. The Journal of Sexual Medicine 2010;7(4 Pt 2):1668-86.
13. Waldinger MD. Towards evidence-based drug treatment research on premature ejaculation: a critical evaluation of methodology. International Journal of Impotence Research 2003;15(5):309-13.
14. Simsek A, Kirecci SL, Kucuktopcu O, et al. Comparison of paroxetine and dapoxetine, a novel selective serotonin reuptake inhibitor in the treatment of premature ejaculation. Asian Journal of Andrology 2014;16(5):725-7.
15. McMahon CG, McMahon CN, Leow LJ, Winestock CG. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: a systematic review. BJU International 2006;98(2):259-72.
16. Chen J, Keren-Paz G, Bar-Yosef Y, Matzkin H. The role of phosphodiesterase type 5 inhibitors in the management of premature ejaculation: a critical analysis of basic science and clinical data. European Urology 2007;52(5):1331-9.
17. Lee WK, Lee SH, Cho ST, et al. Comparison between on-demand dosing of dapoxetine alone and dapoxetine plus mirodenafil in patients with lifelong premature ejaculation: prospective, randomized, double-blind, placebo-controlled, multicenter study. The Journal of Sexual Medicine 2013;10(11):2832-41.
18. Polat EC, Ozbek E, Otunctemur A, et al. Combination therapy with selective serotonin reuptake inhibitors and phosphodiesterase-5 inhibitors in the treatment of premature ejaculation. Andrologia 2014 [Epub ahead of print].
19. McMahon CG, Giuliano F, Dean J, et al. Efficacy and safety of dapoxetine in men with premature ejaculation and concomitant erectile dysfunction treated with a phosphodiesterase type 5 inhibitor: randomized, placebo-controlled, phase III study. The Journal of Sexual Medicine 2013;10(9):2312-25.
20. Porst H, McMahon CG, Althof SE, et al. Baseline characteristics and treatment outcomes for men with acquired or lifelong premature ejaculation with mild or no erectile dysfunction: integrated analyses of two phase 3 dapoxetine trials. The Journal of Sexual Medicine 2010;7(6):2231-42.
21. Narayan P, Lepor H. Long-term, open-label, phase III multicenter study of tamsulosin in benign prostatic hyperplasia. Urology 2001;57(3):466-70.
22. Yeni E, Unal D, Verit A, Gulum M. Minimal transurethral prostatectomy plus bladder neck incision versus standard transurethral prostatectomy in patients with benign prostatic hyperplasia: a randomised prospective study. Urologia Internationalis 2002;69(4):283-6.
23. Tuhkanen K, Heino A, Ala-Opas M. Contact laser prostatectomy compared to TURP in prostatic hyperplasia smaller than 40 ml. Six-month follow-up with complex urodynamic assessment. Scandinavian Journal of Urology and Nephrology 1999;33(1):31-4.
24. Woo HH, Bolton DM, Laborde E, et al. Preservation of sexual function with the prostatic urethral lift: a novel treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia. The Journal of Sexual Medicine 2012;9(2):568-75.
25. Fedder J, Kaspersen MD, Brandslund I, Hojgaard A. Retrograde ejaculation and sexual dysfunction in men with diabetes mellitus: a prospective, controlled study. Andrology 2013;1(4):602-6.
26. Chehensse C, Bahrami S, Denys P, et al. The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update 2013;19(5):507-26.
27. Ohl DA, Quallich SA, Sonksen J, et al. Anejaculation: an electrifying approach. Seminars in Reproductive Medicine 2009;27(2):179-85.
Declaration of competing interests: None declared.






