The current therapeutic ratio for radical therapy in many men with localised prostate cancer is not ideal. For a significant side-effect profile, there seems to be a small survival benefit over a 10-15 year period. A strategy that might balance this therapeutic ratio is focal therapy (FT) which involves treating areas of cancer, with a margin, rather than the whole prostate. As a concept, we apply it to almost every single solid organ cancer. Yet, it seems to trigger controversy in prostate cancer.
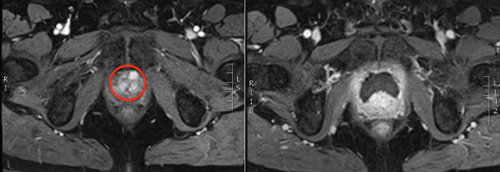
Figure 1: Left – red circle highlighting anterior tumour, right –
post cryotherapy dynamic contrast enhanced MRI showing ablated area.
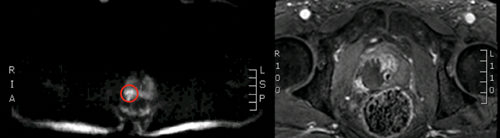
Figure 2: Left - red circle highlighting posterior tumour, Right –
post HIFU dynamic contrast enhanced MRI showing ablated area.
Focal therapy developed from the index lesion theory of prostate cancer. In essence, the largest and highest grade lesion within the prostate almost always drives the natural history of the disease (Figure 1 and 2). The technological evolution and innovation needed to facilitate focal therapy was firstly, the ability to accurately detect, localise and characterise areas of significant cancer (often the index lesion); and secondly, the ability to selectively ablate prostate tissue. In this article, we summarise the results of these advancements, along with the latest clinical data and build the argument that FT is ready for prime time.
Basis behind focal therapy?
It is now established that prostate cancer is commonly multifocal. However, the majority of these multifocal tumours are of low volume and low grade [1]. The index lesion is based upon the monoclonal origin theory that disease progression is related to one tumour / cell which often resides in the largest and highest grade lesion [2]. Although there are a handful of case reports documenting that low grade tumours may lead to disease progression, these are not the norm and large retrospective series have confirmed that low grade cancer has a very low to zero potential for progression and metastases [3-5]. In addition, results from studies such as PIVOT highlight the fact that low grade cancer does not need active treatment [6]. In addition, the largest randomised controlled trial (RCT) of prostate cancer, PROTECT, has recently published its results and showed no difference in 10-year survival between active surveillance, radical prostatectomy and radiotherapy in men with largely low-risk prostate cancer diagnosed by transrectal ultrasound (TRUS) biopsy only [7]. The concept has now progressed and we often talk of clinically significant disease as men who have two or more lesions which are large and high grade [8] – nonetheless, the majority have one index lesion and two to three small low grade cancers.
How can we define clinically significant disease?
Grade is clearly important but the percentage of pattern four also seems to be predictive of outcomes [9,10]. Combined with grade, tumour volume is also predictive for worse pathologic factors such as capsular invasion, seminal vesicle invasion and metastases – the cut-off of 0.5cc as a volume threshold proposed by Stamey was a rather arbitrary one but has been commonly adopted [8]. Recent data from the European Screening Study has placed the volume threshold at 1.3cc if a lesion is pure Gleason 6 on whole-mount.
Attempts have been made to predict tumour volume on targeted biopsy. Computer modelling has led to the derivation of the UCL definitions of maximum cancer core length (MCCL) of ≥6mm to predict a tumour volume of >/=0.5cc with 95% sensitivity and MCCL of ≥4mm to predict a tumour volume of 0.2cc [11].
The currently accepted definition for clinical significant cancer is still not well defined but it is clear that our current definitions using conservative thresholds, such as tumour volume ≥0.2cc or >/=0.5cc, presence of any pattern four, cancer core lengths of 4 or 6mm, may still lead to significant over-treatment but do need to be balanced with the risk of under-treatment [12].
How can we localise cancer?
TRUS biopsy has consistently been shown to be inadequate at defining the index lesion within the prostate when compared to a transperineal template 5mm mapping biopsy (TPM) which can provide the most accurate disease stratification and localisation needed for patients undergoing FT [13,14]. However, the next big step for FT occurred when multi-parametric MRI (mpMRI) became a mainstream tool.
Over the past decade mpMRI has developed significantly, and results from large scale studies confirm that tumours can be visualised within the prostate with a high degree of accuracy. Results both from the literature and recently from the PROMIS trial have shown that mpMRI has sensitivities above 90% and negative predictive values of 80-95%. The ability to rule out significant cancer is crucial for a strategy that aims to leave tissue untreated. Whilst some argue this NPV level is not sufficient, one must remember that currently we use a test, TRUS-biopsy, which has an NPV of 60-70%, to reassure men when their biopsy is negative and often discharge them. Whilst no test is going to be 100% accurate, mpMRI has characteristics that make it a favourable tool for delivery of focal therapy [15].
Focal therapy clinical results
Various energy modalities exist to selectively ablate tissue in the prostate. In pole position are certainly high intensity focused ultrasound (HIFU) and cryotherapy whilst others such as, irreversible electroporation, radiofrequency ablation, magnetic thermotherapy, convective thermal water vapour, injectable toxins, focal brachytherapy, are still in the early phases of evaluation for clinically significant disease.
Clinical outcome papers started appearing in the mid 2000s, initially for focal cryotherapy and then focal HIFU. What was clear very early on was that genitourinary function was preserved in the vast majority of men and early disease control appeared promising, leading to numerous prospective developmental trials. A recent systematic review of 2350 cases of men undergoing focal therapy from 30 studies showed pad-free continence rates of 95-100% and preservation of erectile function in 54-100%. With follow-up ranging from 0 to 11 years, in the primary setting biochemical disease-free survival (bDFS) was 60–83%, whilst post-treatment histological absence of clinically significant cancer ranged from 83-100%. It must be noted that 50% of these men were intermediate or high-risk. In the UK, three early phase trials of 20, 41 and 56 men showed that hemiablation, focal ablation and index lesion ablation were safe, feasible, conferred low toxicity and acceptable rates of disease control in the short-term [16-18].
Ward and Jones presented data from the US Cryo On-Line Database (COLD) registry on focal cryotherapy in 1160 men. Their results showed a bDFS (ASTRO defined) of 75.7% at three-years. Prostate biopsy was generally only performed in those with suspected recurrence in 164/1160 of patients and was positive in 43/164 (26.3%) but in only 3.7% (43/1160) overall. The pad-free continence rate (any pad use) was 98.4% and preservation of erectile function was 58.1%. Rectourethral fistula occurred in only one patient [19].
Vascular-targeted photodynamic therapy (PDT / VTP) is an energy modality that has also been evaluated in a multicentre European RCT; however, the population selected was very low-risk and not relevant in this current era in which active surveillance is accepted as a safe strategy. Certainly focal therapy is not an alternative to active surveillance or a treatment for anxiety which can be physician modulated at the time of giving a diagnosis.
National Health & Care Excellence (NICE) IPG guidance 118 and 424 permit focal HIFU and focal cryotherapy, provided special measures are in place for providing information to patients, consenting, multidisciplinary team (MDT) discussion of all cases and entry of all cases into a registry. The UK Focal Therapy User Group (current Chair, Hashim Ahmed) aims to ensure that high quality training, quality control, data collection and delivery of registry publications occur in the UK whilst also encouraging ongoing research.
The Group has recently presented clinical outcomes and functional data from 625 men undergoing focal HIFU across a number of centres and users in the UK. These were collected into a NICE-compliant academic registry with all cases consecutively entered. Within this cohort 80 (13%) had low-risk, 491 (81%) had intermediate-risk and 39 (6%) had high-risk disease. Median follow-up was 56 (IQR 33-70) months. Overall re-treatment rate with further FT was 20% whilst transition to radical therapy occurred in 7% and systemic therapy in 1%. The metastasis-free survival, cancer-specific survival and overall-survival at five years were 97%, 100% and 99%, respectively. The pad-free continence rate (any pad use) was 97% and preservation of erectile function was 84% [20]. The out-of-field (untreated tissue) de novo disease or progression occurred in only 2-3%, pointing to the safety of leaving untreated tissue and cancers on surveillance after focal therapy and adding more credence to the index lesion concept.
Is focal therapy ready for prime time?
Recent results from large FT series of patients with largely intermediate-risk disease, which would be deemed clinically significant and warranting treatment, show comparable disease control data in the medium term to the other whole-gland treatments. Although long-term data is clearly needed, the current over-treatment, treatment-related harms and minimal impact on survival that radical therapy confers, points to the need to ensure patients are fully informed of alternatives. As an alternative treatment, FT allows patients a 90% probability of avoiding radical therapy for up to five years whilst conferring a 98% chance of avoiding pad-requiring incontinence and an 85-95% probability of keeping erectile function sufficient for penetrative sex.
FT is also not aiming to replace all other treatments for prostate cancer but is one more clinically proven treatment in the clinician’s arsenal when tackling a very common cancer. A commonly asked question is “why are there so many different treatments for men with prostate cancer?” The answer is that no one treatment is perfect. The ideal treatment would remove all the cancer in one session, removing risk of any subsequent progression and thus alleviating the anxiety associated with life-long follow-up in addition to providing zero side-effects and having no impact on a patient’s quality of life. Unfortunately, this does not exist and consultations with men regarding prostate cancer are centred around oncological outcomes, treatment morbidity and quality of life changes associated with each treatment option. Within this context no one treatment clearly excels over the other. Each, including FT, has a role to play in the patients’ treatment journey and ultimately the choice belongs to the fully informed patient.
References
1. Karavitakis M, Winkler M, Abel P, et al. Histological characteristics of the index lesion in whole-mount radical prostatectomy specimens: implications for focal therapy. Prostate Cancer and Prostatic Diseases 2011;14(1):46-52.
2. Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009;15(5):559-65.
3. Haffner MC, Mosbruger T, Esopi DM, et al. Tracking the clonal origin of lethal prostate cancer. The Journal of Clinical Investigation 2013;123(11):4918-22.
4. Ross HM, Kryvenko ON, Cowan JE, et al. Do adenocarcinomas of the prostate with Gleason score (GS) </=6 have the potential to metastasize to lymph nodes? The American Journal of Surgical Pathology 2012;36(9):1346-52.
5. Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. The Journal of Urology 2011;185(3):869-75.
6. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England Journal of Medicine 2012;367(3):203-13.
7. Hamdy FC, Donovan JL, Lane JA, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. The New England Journal of Medicine 2016 [Epub ahead of print].
8. Bostwick DG, Graham SD Jr, Napalkov P, et al. Staging of early prostate cancer: a proposed tumor volume-based prognostic index. Urology 1993;41(5):403-11.
9. Epstein JI, Zelefsky MJ, Sjoberg DD, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. European Urology 2016;69(3):428-35.
10. Choy B, Pearce SM, Anderson BB, et al. Prognostic significance of percentage and architectural types of contemporary gleason pattern 4 prostate cancer in radical prostatectomy. The American Journal of Surgical Pathology 2016;40(10):1400-6.
11. Ahmed HU, Hu Y, Carter T, et al. Characterizing clinically significant prostate cancer using template prostate mapping biopsy. The Journal of Urology 2011;186(2):458-64.
12. Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. The American Journal of Surgical Pathology 2016;40(2):244-52.
13. Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. Journal of Clinical Oncology 2009;27(26):4321-6.
14. Hu Y, Ahmed HU, Carter T, et al. A biopsy simulation study to assess the accuracy of several transrectal ultrasonography (TRUS)-biopsy strategies compared with template prostate mapping biopsies in patients who have undergone radical prostatectomy. BJU International 2012;110(6):812-20.
15. Ahmed HU, Brown LC, Kaplan RS, et al. The PROMIS study: A paired-cohort, blinded confirmatory study evaluating the accuracy of multi-parametric MRI and TRUS biopsy in men with an elevated PSA. Journal of Clinical Oncology 2016;34(suppl; abstr 5000).
16. Ahmed HU, Dickinson L, Charman S, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. European Urology 2015;68(6):927-36.
17. Ahmed HU, Freeman A, Kirkham A, et al. Focal therapy for localized prostate cancer: a phase I/II trial. The Journal of Urology 2011;185(4):1246-54.
18. Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. The Lancet Oncology 2012;13(6):622-32.
19. Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo On-Line Database (COLD) Registry. BJU International 2012;109(11):1648-54.
20. Guillaumier S, Charman S, van der Meulen J, et al. MP18-08 focal HIFU for treatment of localised prostate cancer: a multi-centre registry experience. The Journal of Urology 2016;195(4):e195.
Declaration of competing interests:
TT Shah would like to acknowledge funding from the St Peters Trust for clinical research and has received funding for conference attendance from Astellis, Ferring, and Galil Medical.
HU Ahmed would like to acknowledge funding from the Medical Research Council (UK), the Pelican Cancer Foundation Charity, Prostate Cancer UK, St Peters Trust Charity, Prostate Cancer Research Centre the Wellcome Trust, National Institute of Health Research-Health Technology Assessment Programme, and the US National Institute of Health-National Cancer Institute. HU Ahmed also receives funding from USHIFU, Trod Medical and Sophiris Biocorp for clinical trials.






