Men with localised prostate cancer have traditionally required whole gland treatment involving radical prostatectomy or radical radiation treatment, independent of disease location and size.
Increasing evidence supports the use of active treatment only in those men diagnosed with prostate cancer that is at intermediate or high risk of progression, while low risk men should be offered active surveillance [1,2].
Though such treatment options offer progression-free, and some survival benefit, they can cause a detrimental impact on quality of life. Whole gland treatments were traditionally required due to limitations in diagnostic accuracy. However, with the transition from random transrectal ultrasound (TRUS) guided biopsy, to the more accurate MRI guided approach, clinicians can be more confident in the risk strata and location of cancer within the gland [3].
Focal therapy
As we now better understand the drivers of metastatic disease caused by an ‘index lesion’ in most cases of non-metastatic prostate cancer, which can be targeted with focal ablative treatment, whilst monitoring untreated areas of clinically insignificant cancer [4,5]; it is estimated that 8000-10,000 men every year are suitable for focal therapy in the UK. Indeed, patients are willing to trade a small reduction in cancer control and survival for better functional outcomes and improved quality of life [6]; from large cohort studies of focal therapy, it does not seem necessary that they would need to compromise on survival. Illustrations of disease localisation and characteristics suitable for focal therapy are demonstrated in Figure 1.
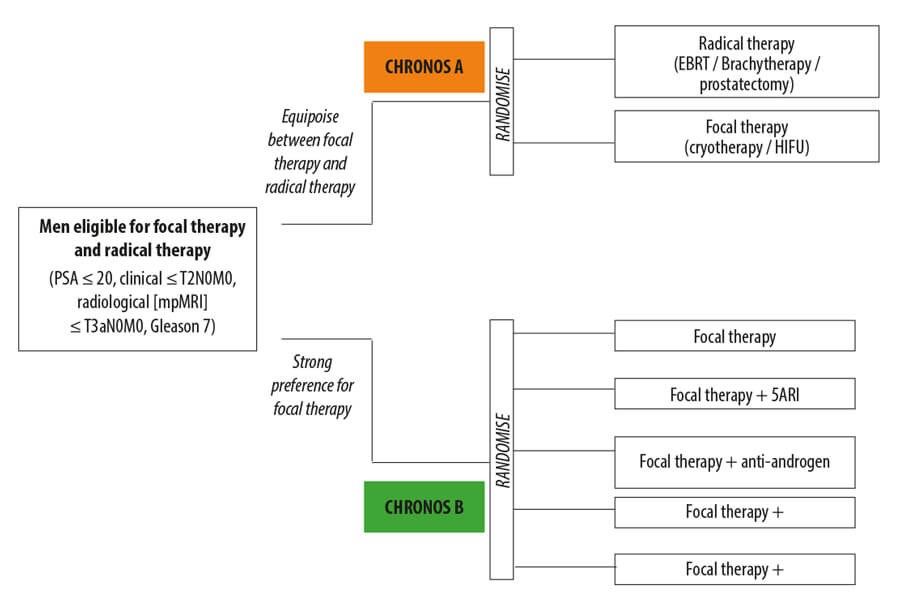
Figure 1: IP4-CHRONOS (NCT04049747) trial design.
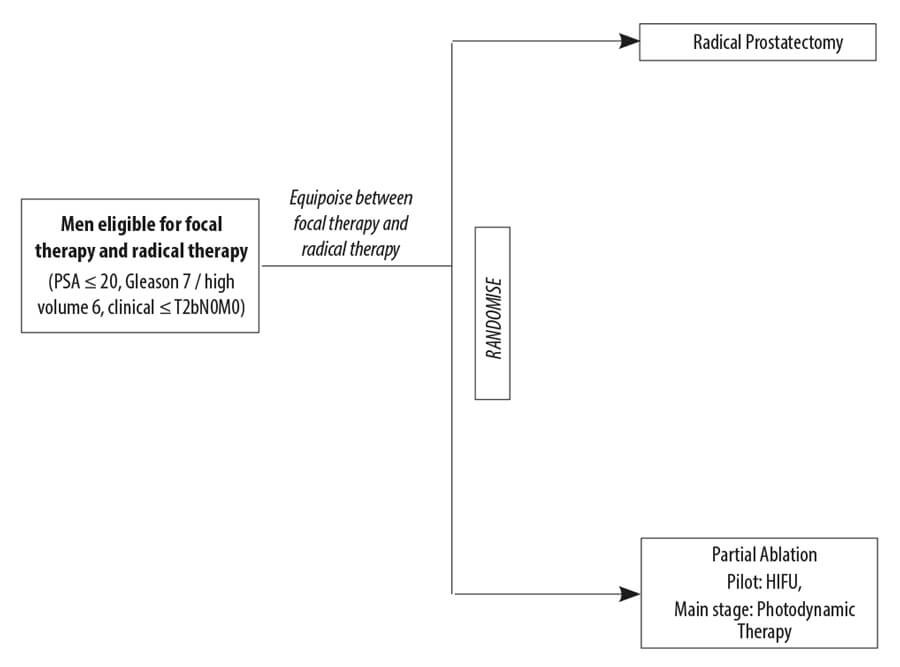
Figure 2: PART (ISRCTN99760303) trial design.
The National Institute for Health and Care Excellence (NICE) permits the use of focal therapy to manage localised prostate cancer in specific circumstances such as a registry or within clinical trials [7,8]. Observational studies have reported overall failure free survival of 88% at five years following focal high intensity focused ultrasound (HIFU) and 90.5% at three years for cryotherapy. Focal therapy is a strategy that includes up to two treatment sessions and 20-25% of patients require two sessions over the course of five years [9]. Incontinence is 0-2% (defined by a much stronger endpoint of any pad use) and erectile dysfunction of 5-15% with rectourethral fistula risk at 0-0.2%.
Nonetheless, concern has been raised about the lack of long-term comparative outcomes of focal versus whole gland treatment options [10]. It is debateable whether a randomised controlled trial (RCT) evaluating long-term mortality and metastases could ever be delivered considering the non-inferiority design that requires a number of thousands of patients and at least 15 years to complete [11].
Not surprisingly, randomised trials have been sparse. To date, there have been about 11 RCTs in the localised disease space that have closed early; a number of these have been in the UK. The lack of physician and patient equipoise to even randomise between two radical therapies indicates the challenges that are faced.
To date, the only completed phase II RCT evaluated the use of photodynamic therapy (PDT) in patients with either low or very low risk disease [9,12,13]. Though it observed only 6% of patients transitioning to whole gland treatment after PDT compared to 29% active surveillance undergoing whole gland treatment, the study was heavily criticised for only including patients that would not typically have been offered whole gland treatment [14]. Furthermore, whilst an MRI scan was carried out within the trial, it was not permitted to conduct additional targeted and systematic biopsies prior to randomisation. This artificially enriched the event rate for misclassification of the initial transrectal systematic (non-MRI based) biopsy within the active surveillance arm.
Trials in focal therapy
Novel trial designs that reflect current patient and physician equipoise are required to test whether randomisation between focal and radical therapies are possible [15].
There are notable efforts in this area
The phase II RCT called PART (Partial ablation versus radical prostatectomy in intermediate-risk prostate cancer ISRCTN99760303, funder: NIHR HTA) led by Professor Hamdy has proven a degree of recruitment feasibility in 2018, randomising men between radical prostatectomy and HIFU. However, even in this feasibility study, the recruitment period had to be extended and within the radical arm, there was 20% non-compliance to radical prostatectomy indicating patient non-acceptance of their random allocation. The main stage is due to open soon, and will now be evaluating PDT (using Tookad Soluble) against radical therapy (surgery or radiation), presumably because men would not otherwise be able to access PDT in standard care following the rejection by NICE to approve use of Tookad Soluble in the NHS. A further internal feasibility phase is built in to assess recruitment and compliance.
The IP4-CHRONOS (Comparative Health Research Outcomes of NOvel Surgery in Prostate Cancer NCT04049747; funder Prostate Cancer UK) attempts to adopt a novel trial design that reflects physician and patient equipoise. There are two linked RCTs. The first, CHRONOS-A, will evaluate the feasibility of recruitment to focal therapy vs. radical therapy (surgery or radiation). The second, CHRONOS-B will assess whether neoadjuvant medications, such as androgen deprivation therapy, might improve current outcomes of focal therapy further. CHRONOS-B is a multi-arm multi-stage design similar to the STAMPEDE.
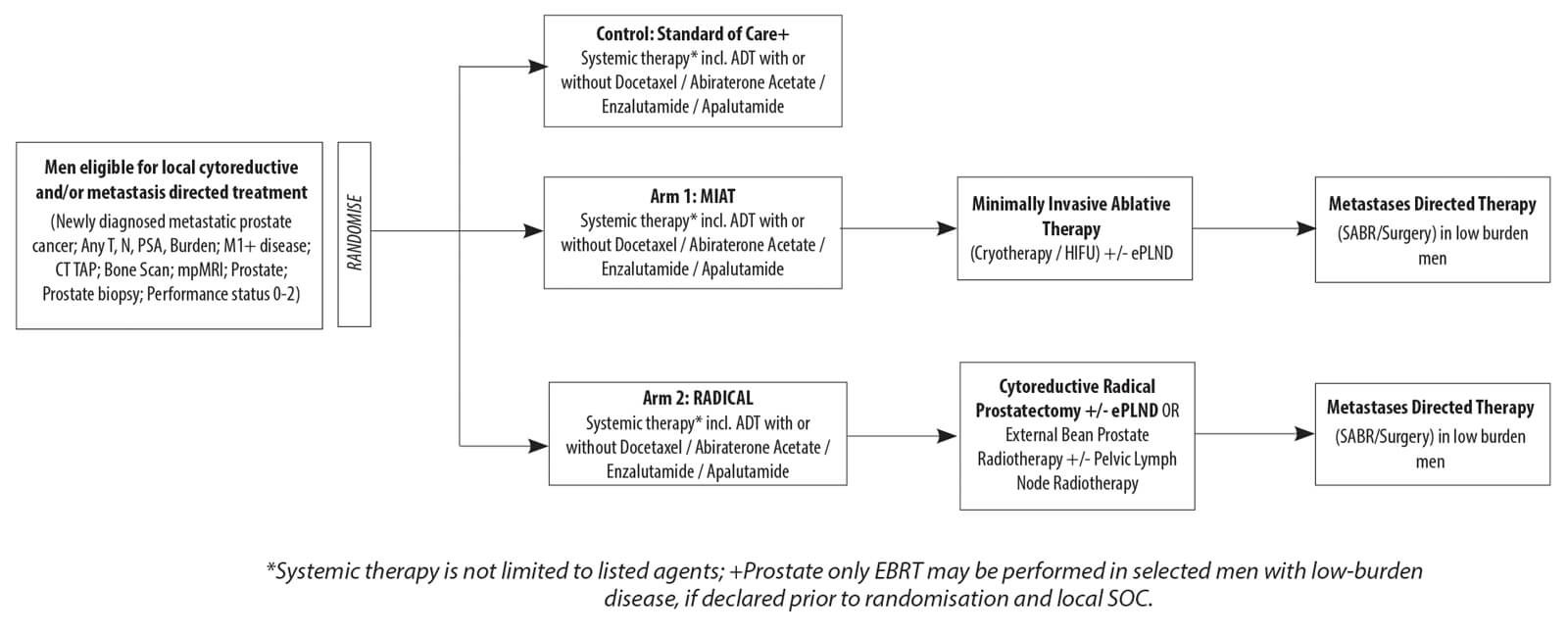
Figure 3: IP2-ATLANTA (NCT03763253) trial design.
The IP2-ATLANTA (Adjuvant Treatments to the Local Tumour for Metastatic Prostate Cancer: Assessment of Novel Treatment Algorithms NCT03763253; funder Wellcome Trust) will recruit newly diagnosed metastatic patients and randomised between standard of care alone, or additional radical local treatment (surgery or radiation) or local ablative treatment (cryotherapy or HIFU).
Medium-long term oncological and safety outcomes from trials such as ATLANTA, CHRONOS and PART will take many years to report. The observational phase II INDEX trial is currently in follow-up and due to report early outcomes in the next one to two years. Further feasibility and early oncological outcomes following focal ablative salvage treatment in men with radio-recurrent cancer is due to be reported by the FORECAST (FOcal RECurrent Assessment and Salvage Treatment NCT01883128) trial and a randomised trial is currently being planned in the setting of recurrence after radiotherapy.
Whilst feasibility of randomised trials is still in question and further tests of their delivery are awaited, longitudinal evaluation of cancer control following focal therapy using HIFU and cryotherapy continue to support their role as a standard option during counselling for patients with eligible disease.
References
1. Wilt TJ, Jones KM, Barry MJ, et al. Follow-up of prostatectomy versus observation for early prostate cancer. N Engl J Med 2017;377(2):132-42.
2. Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med 2014;370(10):932-42.
3. Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. The Lancet 2017;389(10071):815-22.
4. Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009;15(5):559-65.
5. Ahmed HU, Dickinson L, Charman S, et al. Focal ablation targeted to the index lesion in multifocal localised prostate cancer: a prospective development study. Eur Urol 2015;68(6):927-36.
6. Watson V, McCartan N, Krucien N, et al. Evaluating the trade-offs men with localised prostate cancer make between the risks and benefits of treatments: the COMPARE study. The Journal of Urology 2020;204(2):273-80.
7. Excellence, N.I.f.H.a.C. Focal therapy using cryoablation for localised prostate cancer: IPG 423. 2012.
8. Excellence, N.I.f.H.a.C. Focal therapy using high-intensity focused ultrasound for localised prostate cancer. N.I.f.H.a.C. Excellence, Editor. 2012.
9. Guillaumier S, Peters M, Arya M, et al. A multicentre study of 5-year outcomes following focal therapy in treating clinically significant nonmetastatic prostate cancer. Eur Urol 2018;74(4):422-9.
10. van der Poel HG, van den Bergh RCN, Briers E, et al. Focal therapy in primary localised prostate cancer: the European Association of Urology position in 2018. Eur Urol 2018;74(1):84-91.
11. Ahmed HU, et al. Can we deliver randomized trials of focal therapy in prostate cancer? Nat Rev Clin Oncol 2014;11(8):482-91.
12. Azzouzi A-R, Vincendeau S, Barret E, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. The Lancet Oncology 2017;18(2):181-91.
13. Shah TT, Peters M, Eldred-Evans D, et al. Early-medium-term outcomes of primary focal cryotherapy to treat nonmetastatic clinically significant prostate cancer from a prospective multicentre registry. Eur Urol 2019;76(1):98-105.
14. Taneja SS. Re: Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. The Journal of Urology 2017;198(2):255-7.
15. Reddy D, Shah TT, Dudderidge T, et al. Comparative healthcare research outcomes of novel surgery in prostate cancer (IP4-CHRONOS): a prospective, multi-centre therapeutic phase ii parallel randomised control trial. Contemp Clin Trials 2020;93:105999.
Declaration of competing interests:
DR’s research is funded by Prostate Cancer UK and she has received reimbursement from SonaCare Medical for attending conferences.
HUA’s research is supported by core funding from the United Kingdom’s National Institute of Health Research (NIHR) Imperial Biomedical Research Centre. HUA currently receives funding from the Wellcome Trust, Medical Research Council (UK), Cancer Research UK, Prostate Cancer UK, The Urology Foundation, BMA Foundation, Imperial Health Charity, NIHR Imperial BRC, Sonacare Inc., Trod Medical and Sophiris Biocorp for trials in prostate cancer. HUA was a paid medical consultant for Sophiris Biocorp in the previous three years. HUA is a proctor for HIFU and cryotherapy and is paid for training other surgeons in these procedures. HUA is also a proctor for Rezum for the treatment of benign prostate hyperplasia.





