The first article in this series defined frailty and introduced the concept and importance of identifying patients living with frailty who undergo surgery, including those undergoing urological procedures. In the second part of this series we outline how to identify frailty and what impact this can have on the decision-making process for both surgeons and their patients.
Assessing for frailty
Assessing for frailty in the clinical setting remains somewhat controversial, with no agreed gold standard measure. More than 60 screening tools have been described, however many issues with these exist, namely, disagreements between scores, lack of validation in a healthy population and a disproportionate focus on comorbidities [1,2].
The Rockwood Clinical Frailty Scale (CFS – can be viewed at https://static1.squarespace.com/static/5b5f1d4e9d5abb9699cb8a75/t/5e7885fb34a6bb7701bd2713/1584956923657/Rockwood+CFS.pdf) is one of the best-known frailty screening tools and its use in the perioperative setting is recommended by the British Geriatric Society / Centre for Perioperative Care [3]. The tool gained traction during the COVID-19 pandemic, where the National Institute for Health and Care Excellence (NICE) recommended that all patients admitted to the acute hospital setting aged >65 years were screened to ensure holistic care for the unwell and frail patient [4]. It reliably predicts outcomes, including length of stay, likelihood of complications and in hospital mortality, however there are limited data on its use in patients undergoing urological surgery [5,6].
The CFS is a judgement tool which rates a patient from one (very fit) to nine (terminally ill), based on their level of function and the impact of chronic health conditions on their daily activities [7]. In the acute setting the patient is scored on how they were two weeks prior to assessment to help gauge a more accurate baseline. The system is validated only for patients aged >65 years and provides a quick and easy screening tool which is widely available across the NHS and can easily be administered by the surgical team with minimal training. Ken Rockwood provides some simple tips to help users become more confident when assessing for frailty (https://www.scfn.org.uk/clinical-frailty-scale).
The Edmonton Frail Scale (EFS) is a multi-domain alternative to the CFS, which assesses nine frailty domains (e.g. nutrition, medication use, functional independence) [8]. In total patients are rated from 0 (not frail) to 17 (severe frailty). Given this multidimensional approach, the EFS is more suited to the elective outpatients setting as it provides a more granular assessment of the key domains of frailty and identifies common areas that are open to optimisation preoperatively. Whilst more detailed than the CFS, the EFS can be performed in a matter of minutes and has been validated for use by healthcare professionals with no formal training in geriatric medicine [8]. Just like the CFS, there is scant evidence on its use in the context of urological surgery [9].
Within primary care, the electronic Frailty Index (eFI) uses existing patient records to automatically generate a frailty score to help guide clinicians to the likelihood of a patient living with frailty. Real-world analysis of over 930,000 individuals identified that the eFI is associated with both short- and long-term mortality and unplanned hospitalisations [10]. Whilst of use for screening patients within the community setting, the eFI has little application to acute care and also focuses heavily on recorded comorbidities rather than true domains of frailty.
Other ways to identify frailty involve focusing on specific ‘syndromes’ of frailty, famously termed the ‘geriatric giants’. These include falls, immobility, incontinence and delirium. Whilst these don’t quantify the severity of frailty, they can help to guide a clinician to the possibility their patient is frail. Common individual assessments include the Timed Up and Go Test (TUGT), gait speed, grip strength and patient reported outcome scores, such as the PRISMA 7 Questionnaire.
Overall, whilst there are many scoring systems to use, emphasis should be placed on consistency of use. Given its ease of use and brevity, the CFS seems a logical ‘first-line’ tool to use by the surgical team.
Assessing preoperative risk
Increasing age, multi-morbidity and frailty are independent predictors of adverse outcomes, however it should be stressed that frailty itself is not the same as surgical risk. Therefore, utilising a surgical risk tool in combination with frailty assessment, clinical judgement and the patient’s own beliefs and values is critical to ensuring informed shared decision-making.
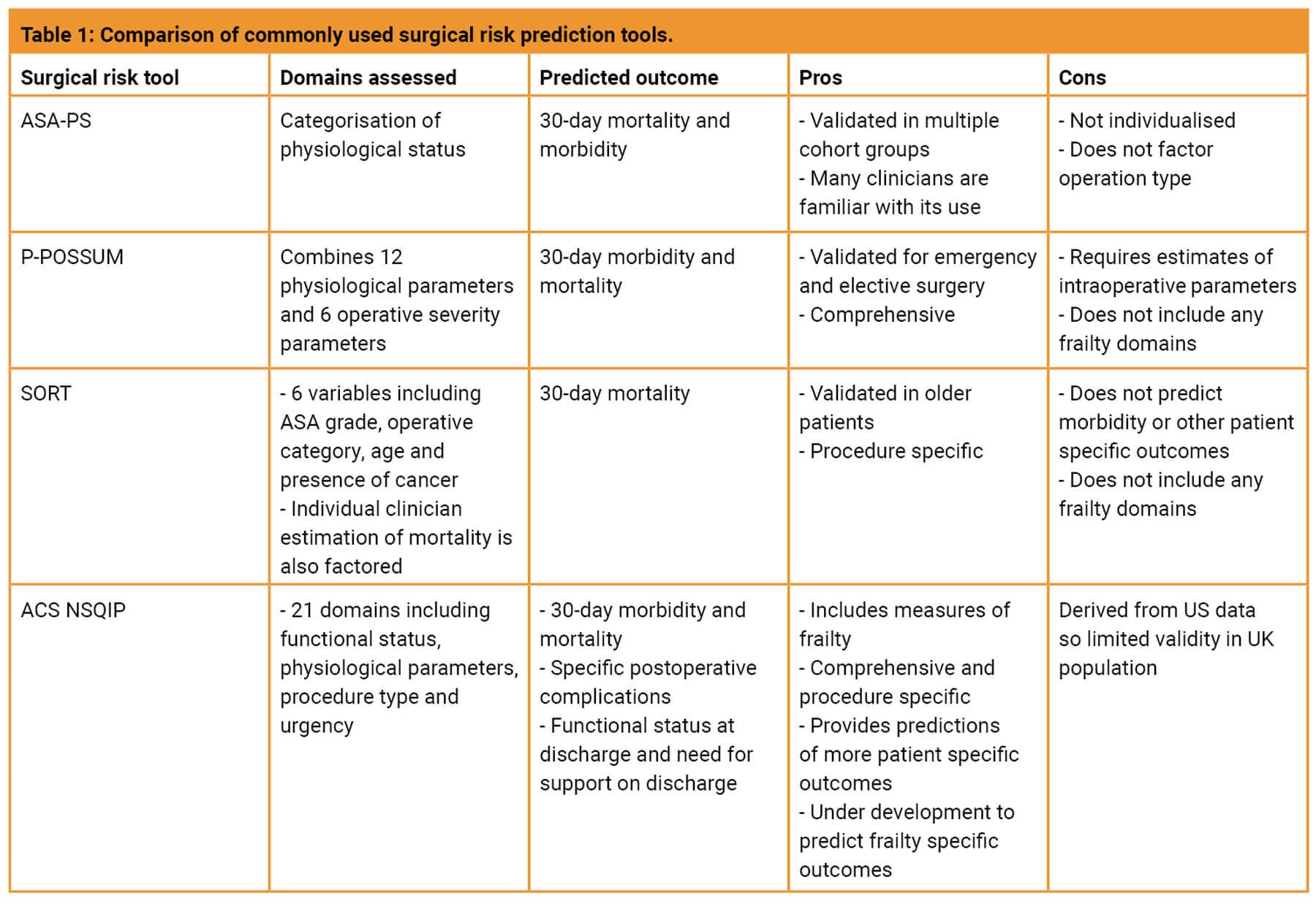
A comparison of the commonly used risk tools is shown in Table 1. Of those listed, the National Surgical Quality Improvement Program (NSQIP) scores is perhaps of most value for assessing risk in the frail population, given the inclusion of functional status and multi-morbidity in its model and provides predictions of more holistic patient-related outcomes (e.g. length of stay, likelihood of discharge to a rehab or nursing facility). Furthermore, this tool is being developed to help predict specific geriatric outcomes or frailty indicators, including postoperative delirium and functional decline allowing for a better-rounded discussion of an individual’s risks.
Improving patients’ outcomes and experience
Once it has been identified that a patient is living with frailty, the next step for the patient undergoing surgery is to identify potentially modifiable factors that may help improve outcomes as well as focusing on holistic care which can improve overall experience for that individual (Table 2). The nature of these factors and how they can be optimised will to some extent depend on the patient’s acuity and nature of their surgery. In the elective setting much can be achieved in the surgical outpatients and preoperative assessment clinics, however for acute admissions this will largely be undertaken by the surgical team, ideally with help from specialist surgical frailty services.

The Comprehensive Geriatric Assessment (CGA)
For those identified as having a CFS score >5 (mildly frail), it is recommended that a formal comprehensive geriatric assessment (CGA) is undertaken [3]. The CGA provides a comprehensive assessment of the frail patient covering multiple domains, including a physical examination, functional, social and psychological assessment and medication review to help generate a person-specific problem list. Common problems identified include polypharmacy, previously unidentified medical problems, cognitive impairment and risk of delirium. Given the granular nature of the CGA, it is usually performed by a geriatrician-led multidisciplinary team, and often over more than one consultation.
In the elective setting (and to a certain extent in the acute setting), there are common areas to focus to help improve patient outcomes. Improving perioperative malnutrition with readily available and inexpensive supplements can provide potential benefit; in the context of patients undergoing radical cystectomy, oral nutritional supplements helped prevent postoperative sarcopenia and may reduce the risk of major postoperative complications [11].
Delirium
Routine screening for cognitive impairment is useful to help inform the risk of a patient developing delirium during their admission. The importance of delirium cannot be overstated and should not be thought of as simple ‘postop confusion’; in-patients who suffer delirium have a greater than three-fold increased odds of 30-day mortality and double the length of in-patient stay compared to those without [12]. Longer term, in-hospital delirium is also significantly associated with cognitive impairment at three months follow-up [13]. The well-established Mini Mental Status Examination (MMSE), or more detailed Montreal Cognitive Assessment (MoCA) test are readily available tools to detect underlying cognitive impairment, whilst the Delirium Elderly At-Risk (DEAR) tool can be used to reliably predict the risk of postoperative delirium [14].
Reviewing preoperative medications is particularly useful in this group of patients; where possible medications should be rationalised to limit the use of known delirium inducing medications (e.g. opiates, benzodiazepines, etc) and reducing overall anti-cholinergic burden, which itself significantly increases the risk of postoperative delirum [15].
In the postoperative phase identifying delirium, which is sometimes subtle, is of vital importance so that patients can be managed appropriately with delirium-trained specialists. Simple screening tools, such as the 4AT can be easily used by the surgical team.
Function and nutrition
Assessment of preoperative mobility and a history of falls should also be undertaken. A prior history of falls is the leading risk factor for a patient falling again in the future [16]. Preoperative assessment by physio- and occupational therapists focusing on pre-habilitation can help to educate a patient and their families on ways to improve overall mobility, reduce falls risk and can aid planning to proactively address potential barriers to discharge. Programmes championing pre-habilitation and exercise for patients undergoing treatment for cancer, most notably the Wessex Fit-4 Cancer Surgery Trial (Wesfit) and Prehab 4 Cancer, are starting to show promising early results and may lead to major changes in the way we manage patients in the preoperative period [17].
Further to this, poor preoperative nutritional status and sarcopenia can also impact on postoperative outcomes. In the context of patients having treatment for urological cancers, poor preoperative nutritional status (measured using serum albumin concentration) can lead to an almost two-fold increase in the odds of postoperative complications at 30-days [18]. Nutritional assessment, using simple tools such as the Malnutrition Universal Screening Tool (MUST) can easily and quickly be performed in the preoperative setting, with onward referral for dietician input and the provision of nutritional supplements offered to the patient. Interestingly, whilst nutritional supplements offer a simple solution to preoperative malnutritional, their benefits on postoperative outcomes is unclear and to date there are no comparative studies to assess the impact of preoperative nutritional screening on postoperative outcomes [19,20].
Surgical frailty services
Given the increasing number of older individuals undergoing surgery, surgical frailty services are becoming more commonplace [21]. The scope and scale of services may vary between individual trusts. A specialist frailty-focused multidisciplinary team (e.g. geriatrician, nurse specialist, pharmacists, dieticians, physio- and occupational therapists, etc.) can complement the surgical team in the care of frail patients which may involve specialist multidisciplinary preoperative assessment and assisting with inpatient care. Of these services, the Proactive Care of Older People Undergoing Surgery (POPS) team at Guy’s and St Thomas’ NHS Foundation Trust, is the best known and in the context of urology has been shown to significantly reduce postoperative complications and improve inpatient length of stay by 19% [22].
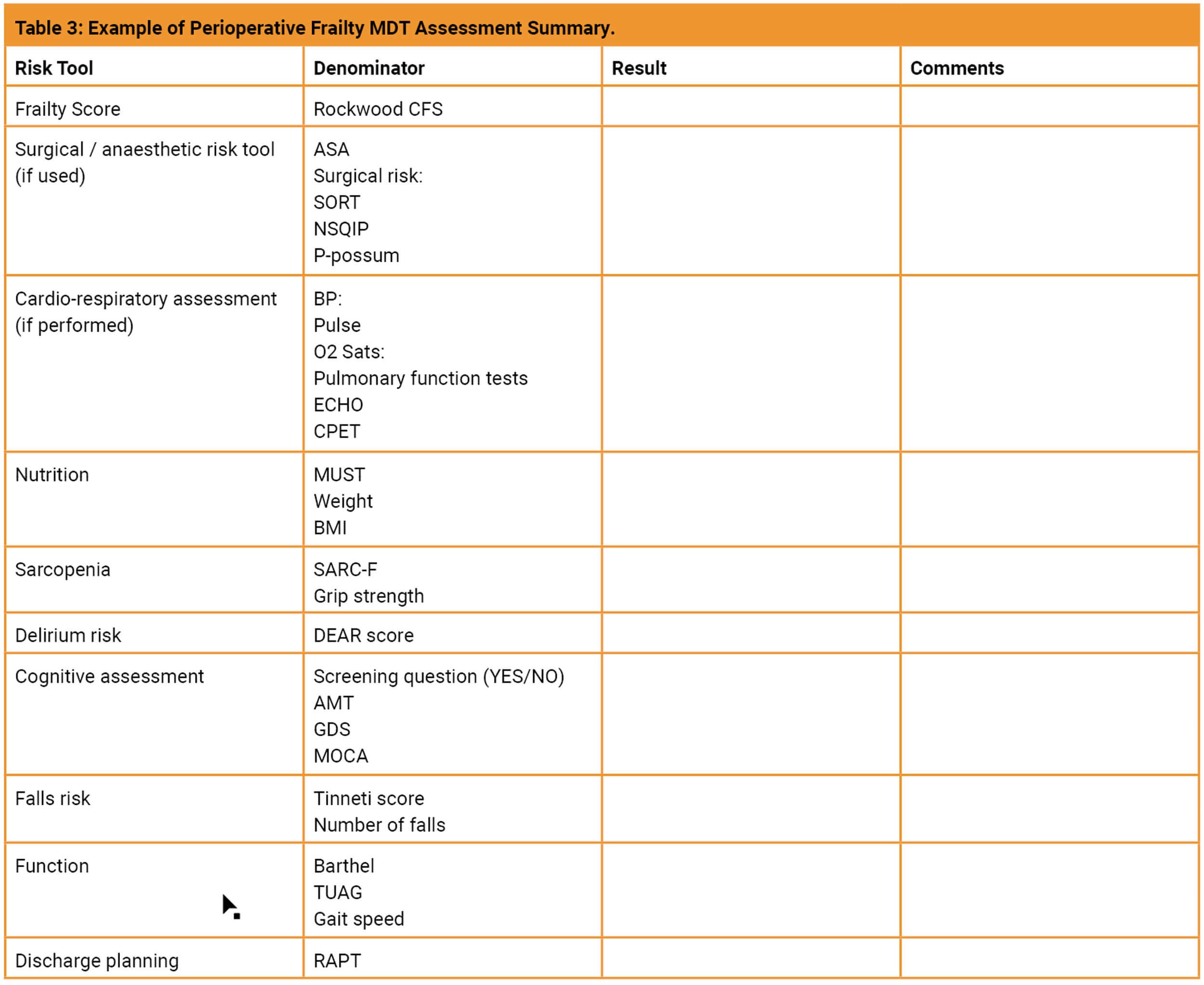
An example of a typical assessment summary produced by University Hospitals Dorset ‘Surgical Frailty’ team in their perioperative clinic is shown in Table 3. A number of assessments are carried out by a multidisciplinary team to produce an individualised problem list and highlight areas of optimisation. From this and following discussion with the patient, their family members or carer and the clinic team, a consensus is reached as to whether it is appropriate to proceed with the proposed procedure, to allow time for further optimisation or if the procedure is to be avoided.
Surgical decision making and pre-emptive communication
The impact of frailty on surgical decision making is complex and it should be stressed that a subjective frailty score alone should not be used to deny a patient surgery. As is often said, a good surgeon understands when, and also when not, to operate. Ultimately the shared decision-making process should include not just discussing the risks of a procedure, but a more holistic dialogue addressing the impact of a patient’s frailty on their expected outcome, postoperative function, likely discharge destination, and importantly, their quality of life and wishes. Shared decision making is endorsed by NHS England and toolkits are available to help guide clinicians [23].
Where surgery is necessary, pre-emptive discussions on escalation plans (particularly in the event of a complication), likely postoperative care needs and expected functional status are of particular benefit. Ideally this should involve family members / carers. When performed well, these discussions can be invaluable in helping to align expectations and preventing misconceptions of what can be achieved.
Concluding remarks
Managing frailty amongst patients undergoing urological procedures is an increasingly common issue, which poses many challenges. Effective assessment and recognition of frailty is essential, and many trusts are now implementing specialist surgical frailty services which can assist surgical teams in the management of these patients. Whilst there is no ‘silver bullet’ to mitigate all the risks associated with frailty, simple interventions and adjustments, as well as effective communication can help both improve outcomes and the experience for patients undergoing urological procedures.
References
1. Houghton JSM, Nickinson ATO, Morton AJ, et al. Frailty Factors and Outcomes in Vascular Surgery Patients: A Systematic Review and Meta-analysis. Annals of Surgery 2020;272:266-76.
2. Aguayo GA, Donneau AF, Vaillant MT, et al. Agreement Between 35 Published Frailty Scores in the General Population. Am J Epidemiol 2017;186:420-34.
3. BGS / CPC. Guideline for the care of people living with frailty undergoing elective and emergency surgery that encompasses the whole perioperative pathway. 2021.
https://www.bgs.org.uk/cpocfrailty
[accessed 29 September 2022].
4. NICE. COVID-19 rapid guideline: critical care in adults [NG159]. 2020.
5. Sun C-Y, Huang C-C, Tsai Y-S, et al. Clinical Frailty Scale in Predicting Postoperative Outcomes in Older Patients Undergoing Curative Surgery for Urologic Malignancies: A Prospective Observational Cohort Study. Urology 2020;144:38-45.
6. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. Qjm 2015;108:943-9.
7. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal 2005;173:489.
8. Rolfson DB, Majumdar SR, Tsuyuki RT, et al. Validity and reliability of the Edmonton Frail Scale. Age and Ageing 2006;35:526-9.
9. Moro FD, Morlacco A, Motterle G, et al. Frailty and elderly in urology: Is there an impact on post-operative complications? Cent European J Urol 2017;70:197-205.
10. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. The Lancet 2013;381:752-62.
11. Ritch CR, Cookson MS, Clark PE, et al. Perioperative Oral Nutrition Supplementation Reduces Prevalence of Sarcopenia following Radical Cystectomy: Results of a Prospective Randomized Controlled Trial. J Urol 2019;201:470-7.
12. Anand A, Cheng M, Ibitoye T, et al. Positive scores on the 4AT delirium assessment tool at hospital admission are linked to mortality, length of stay and home time: two-centre study of 82,770 emergency admissions. Age and Ageing 2022;51:afac051.
13. Goldberg TE, Chen C, Wang Y, et al. Association of Delirium With Long-term Cognitive Decline: A Meta-analysis. JAMA Neurology 2020;77:1373-81.
14. Freter SH, Dunbar MJ, MacLeod H, et al. Predicting post-operative delirium in elective orthopaedic patients: the Delirium Elderly At-Risk (DEAR) instrument. Age and Ageing 2005;34:169-71.
15. Mueller A, Spies CD, Eckardt R, et al. Anticholinergic burden of long-term medication is an independent risk factor for the development of postoperative delirium: A clinical trial. Journal of Clinical Anesthesia 2020;61:109632.
16. Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology 2010;21:658-68.
17. Loughney L, West MA, Dimitrov BD, et al. Physical activity levels in locally advanced rectal cancer patients following neoadjuvant chemoradiotherapy and an exercise training programme before surgery: a pilot study. Perioperative Medicine 2017;6:3.
18. Liu J, Wang F, Li S, et al. The prognostic significance of preoperative serum albumin in urothelial carcinoma: a systematic review and meta-analysis. Biosci Rep 2018;38(4):BSR20180214.
19. NICE. Perioperative care in adults: evidence revew for nutritional screening in pre-operative assessment. 2020.
20. Centre for Operative Care. Impact of perioperative care on healthcare resource use. 2020.
21. Fowler AJ, Abbott TEF, Prowle J, Pearse RM. Age of patients undergoing surgery. Br J Surg 2019;106:1012-18.
22. Braude P, Goodman A, Elias T, et al. Evaluation and establishment of a ward-based geriatric liaison service for older urological surgical patients: Proactive care of Older People undergoing Surgery (POPS)-Urology. BJU International 2017;120:123-9.
23. NHS England. Decision support tools. 2022.
https://www.england.nhs.uk/
shared-decision-making/decision-support-tools/
[accessed 19 September 2022.
Declaration of competing interests: None declared.










