Countless epidemiological studies have established the frequent occurrence of lower urinary tract symptoms (LUTS) and the significant burden these symptoms incur. For the most part of the past three decades, there has been an overwhelming focus on detrusor overactivity (DO) and bladder outlet obstruction (BOO) as the underlying pathophysiological abnormalities giving rise to LUTS.
Consequently there has been a boon in basic and clinical science research, which has given rise to numerous innovations in pharmacological and surgical therapy. By contrast the problem of detrusor underactivity (DU) has been largely neglected, indeed it is salutary to note that the last major advance in management was the introduction of clean intermittent self-catheterisation by the American urologist Jack Lapides some 40 years ago. Recently, there has been resurgence in interest in DU with efforts to better define the problem, understand its epidemiology and develop better diagnostic approaches as well as novel treatments.
Terminology
“When I use a word, it means just what I choose it to mean – neither more nor less.”
This quote from Lewis Carroll's was a favourite of the founding members of the International Continence Society (ICS*), for whom it captured the importance of the principle of using precise and consistent terminology when dealing with lower urinary tract dysfunction. When one surveys even the recent literature it is apparent that there is a confusing plethora of terms in use to describe a problem with bladder emptying that is not due to BOO, such as atonic bladder, areflexic bladder, impaired detrusor contractility, detrusor failure, desensate bladder and chronic retention. The term endorsed by the ICS in the 2002 standardisation report was DU. The term refers to a urodynamic diagnosis (requiring a pressure flow study), which is defined as “a contraction of reduced strength and / or duration, resulting in prolonged bladder emptying and / or failure to achieve complete bladder emptying within a normal time span” [1]. The term DU can be criticised as it suggests a problem with detrusor muscle, whereas the underlying problem may be one of sensory nerves or central neural processing. In addition the definition does not specify what constitutes reduced contraction strength / duration and prolonged bladder emptying, as normal ranges are not established which hampers practical application.
Signs and symptoms
Although there is a lack of prospective studies assessing symptoms in patients with urodynamically confirmed DU, empirical clinical evidence suggests DU gives rise to a mixture of voiding, storage and post-micturition symptoms. The symptoms typically associated with DU are voiding in nature, including reduced flow, prolonged flow, hesitancy and intermittency. Following voiding some patients report a feeling of incomplete bladder emptying which may be related to an elevated post voiding residual urine (PVR). The presence of storage symptoms can be highly variable, and may be related to whether the patient has normal or defective bladder sensation. For example, if the bladder sensation is reduced (due to sensory nerve damage), the individual may have reduced urge to pass urine resulting in infrequent voiding. By contrast if the individual has an elevated PVR in the presence of intact bladder sensation this could result in urinary frequency. In either scenario should the PVR be high enough, overflow or stress incontinence may result.
Given the multitude of possible presenting symptoms, there has been some discussion about whether a particular complex of symptoms is associated with DU, rather analogous to the situation with DO and the overactive bladder (OAB) symptom complex. A symptom complex of ‘underactive bladder’ (UAB) is certainly an appealing idea, as it could potentially allow patients to be diagnosed and treated on the basis of symptoms rather than an invasive urodynamic study. Problematically, the symptoms associated with DU tend to overlap with those associated with other dysfunctions particularly LUTS due to BOO. Thus, developing a definition with sufficient specificity presents a major challenge. Efforts are now underway to develop a working definition of UAB [2] alongside qualitative and quantitative studies aiming to establish the symptoms and frequency of occurrence.
Epidemiology
The absence of a validated definition of UAB along with the inability to diagnose DU without an invasive urodynamic study has meant that large epidemiological studies have not been possible. Although there are several non-invasive proxy measures of DU (e.g. PVR, uroflowmetry), none of these can be reliably used to differentiate patients with DU from those with BOO or both. The available prevalence data has thus been derived from urodynamic studies in patients presenting with LUTS. In this group, DU is found in 9-28% of men under the age of 50, rising to 48% in older men (>70 years) [3]. In older women, DU is found in between 12-45%, peaking in the institutionalised elderly where co-existent DO commonly occurs (detrusor hyperactivity impaired contractility (DHIC)) as described by Resnick and Yalla in 1987 [4].
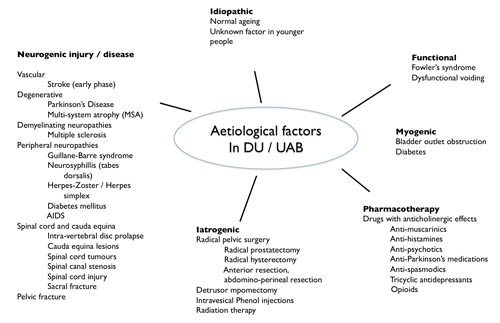
Figure 1: Causes of DU/UAB.
Aetio-pathogenesis
The finding of DU in a wide range of patients would suggest that there are many causes (Figure 1). Much of the literature suggests that DU results as a consequence of the normal ageing process, and whilst this is quite plausible the evidence from the available animal and human studies is contradictory. The causes of DU can be classified as neurogenic or myogenic or a combination of both.
Any process that affects the structure or function of detrusor muscle cells or the surrounding extracellular matrix (ECM) could theoretically lead to an inability to generate or propagate a contraction. An impairment in key cellular processes (e.g. ion storage / exchange, excitation-contraction coupling) could be the cause or, alternatively, it could be due to structural damage to detrusor cells, as described by American pathologist El-badawi who found widespread detrusor muscle cell disruption and degeneration of nerve axons in patients with DU [5]. The constitution and structure of the ECM is essential to the normal biomechanical function of the bladder, processes that affect the ECM such as fibrosis (e.g. due to BOO) could also lead to impairment in bladder contraction.
In terms of neurogenic causes, diseases or injury affecting the motor pathways at the level of cord, nerve roots or pelvic nerves are well recognised as causes of DU. Normal bladder sensory function is also important to the micturition reflex. Sensory nerves monitor the volume of urine in the bladder, not only during the storage phase but also during voiding, providing information as to how empty the bladder is. Sensory nerves from the urethra may also play a contributory role in monitoring urine flow during voiding [6,7]. If sensation is impaired this could lead to a decrease in the strength of detrusor contraction or cause premature termination, resulting in incomplete bladder emptying [8]. Sensory and motor signals are coordinated in the brain stem; functional neuroimaging studies have identified several areas in the cortex and brain stem that become activated during voiding including the insula, the hypothalamus, the periaqueductal grey (PAG) and pontine micturition centre (PMC). Clinical studies however have not always shown a correlation between the site of lesion and DU in diseases such as multiple sclerosis.
It is often considered that DU occurs as a consequence of prolonged BOO in men with benign prostatic enlargement, an assertion that is mainly attributable to the findings of animal studies where a BOO is created by using a constricting metal ring or tie around the urethra. After a variable time period the detrusor decompensates and bladder emptying is impaired. Interestingly, the available clinical evidence does not strongly suggest that prolonged BOO leads to DU. A study from Bristol on 170 men with BOO showed no significant deterioration in urodynamic parameters at a mean follow-up of 13.9 years (no change detrusor pressure at maximum flow) and a reduction in maximum flow rate) of only 1ml/s) [9].
Diagnosis
Pressure flow studies are the only accepted method of diagnosing DU. There are however no widely agreed upon diagnostic criteria; most relate to the strength of detrusor contraction rather than other aspects, such as contraction speed or sustainability, which may also be important.
As a detrusor contraction generates two measurable parameters, pressure and flow, the simplest way to classify whether a detrusor contraction is weak or not, is to use thresholds for the maximal flow (Qmax) and detrusor pressure at maximal flow (Pdet@Qmax). These thresholds are mostly derived from historical series of men undergoing bladder outlet surgery [10,11]. In other groups, such as younger men and women, normal ranges are less clearly established [12-14]. Although this method is simple and easy to use it has two problems. Firstly, it is likely to underestimate the maximal detrusor pressure and hence the maximal contraction strength, due to the nature of the bladder outlet relation (BOR).
The BOR is the inverse relationship between flow and pressure during a given void [15], and can be summarised as when flow is low, pressure is high and vice versa. Hence, Pdet@Qmax represents the point of lowest detrusor pressure. The other issue with this approach is that it fails to consider that the flow rate is also affected by the degree of outlet resistance; the relevance of this is that when pdet@Qmax is low, a low flow could also be attributable to BOO. On the other hand a normal Qmax can result from a weakened outlet even if the pdet@Qmax is low (e.g. post prostatectomy incontinence).

In an effort to more accurately assess contraction strength, methods to assess isovolumetric contraction strength were introduced (summarised in Table 1). There are principally two approaches, using formulae or directly measuring isovolumetric pressure by mechanical obstruction of urine flow (stop test).
It is common for some patients to be unable to void due to anxiety during a standard urodynamic study, which has been attributed to inadequate pelvic floor relaxation and reflex inhibition of detrusor contraction. In such situations it is often easy to differentiate patients with DU by taking a good history of previous voiding symptoms. If there is still doubt then an ambulatory urodynamic study can be useful [16,17].
Management
The overall goals in managing the patient with DU / UAB are to reduce symptoms, improve quality of life and reduce the risk of sequelae of poor bladder emptying (e.g. recurrent urinary tract infection, bladder stones, upper tract damage and skin damage due to overflow incontinence). Due to lack of any effective treatments to improve detrusor contractility, most approaches aim to facilitate bladder drainage using devices or decreasing outlet resistance.
Initially patients with suspected DU should undergo a routine evaluation with bladder diary, digital rectal examination, urinalysis, uroflowmetry, PVR estimation using ultrasound, and neurological assessment (sacral dermatomes, anal tone, bulbocavernosal reflex, lower limb reflexes). In particular attention should be given to identifying medications that may impair bladder contractility (e.g. anticholinergics) or increase outlet resistance (e.g. alpha-adrenoreceptor agonists). It is also important to identify and treat constipation.
Specific behavioural interventions may be useful to improve symptoms and reduce the risk of complications of poor bladder emptying. In infrequent voiders with sensory impairment, scheduled voiding can be helpful. Double voiding can be used a method of improving bladder emptying. Bladder expression methods such as Crede’s manoeuvre are generally not recommended due to the risk of generating high bladder pressures and causing vesicoureteric reflux. In children and adults with dysfunctional voiding due to inadequate pelvic floor relaxation, physiotherapy and biofeedback can be an effective approach.
If post void residuals are problematically high, clean intermittent self-catheterisation (CISC) is the preferable method of bladder drainage with lower rates of infection than indwelling catheters. If CISC is not possible, e.g. due to lack of cognition or dexterity, then a suprapubic catheter is a better long-term option than a urethral catheter.
Currently there are no effective pharmacotherapies to treat DU / UAB. The most commonly studied agents are parasympathomimetics, including direct muscarinic agonists (e.g. bethanechol, carbachol) and anticholinesterases (e.g. distigmine). A systematic review of available studies did not support the use of these agents [18]. The major concern with parasympathomimetics are the dose-dependent side-effects which may be potentially serious, including nausea, bronchospasm, abdominal cramping, diarrhoea, increased salivation, flushing, visual disturbance and rarely severe cardiac depression resulting in cardiac arrest.
Electrical stimulation in various forms has been applied to the treatment of DU of various etiologies, such as the anterior sacral root stimulator for patients with complete spinal cord injury or as sacral neuromodulation in women with non-obstructive urinary retention. A less well-documented technique is intravesical electrotherapy (IVE), introduced by Katona in the late 1950s and mainly studied in paediatric patients [19]. The bladder is filled with saline and current is passed through an electrode at the tip of the catheter. Daily sessions of stimulation are undertaken, usually of one hour or more, with 10-15 sessions considered a trial period. Animal studies suggest that this stimulation leads to activation and upregulation of mechanosensitive bladder afferents; there is however a lack of randomised studies to support the use of IVE in adults with DU.
"The overall goals in managing the patient with DU / UAB are to reduce symptoms, improve quality of life and reduce the risk of sequelae of poor bladder emptying."
The role of bladder outlet surgery in men with DU is a controversial topic with a lack of good quality evidence to guide clinical decision-making. It is important to distinguish between two common scenarios, firstly a patient with DU who has symptoms but low or absent PVR who is not catheter dependent and the patient who is dependent on CISC (or indwelling catheter) for chronic retention. In the former, it is less likely that significant BOO is present as the patient can empty the bladder with low pressure. The limited available evidence suggests that surgery in this scenario is unlikely to result in meaningful improvement in symptoms or urodynamic parameters [20-22]. In men with DU who are catheter dependent, surgery is performed with the aim of reducing outlet resistance to permit emptying without the need for a catheter. Such patients tend to be far less likely to pass postoperative trial without catheter than men with retention and preserved contractility. Although it is difficult to predict outcome in such cases, a proportion of patients will resume spontaneous voiding [23]. Given the lack of any other effective treatment some urologists advocate surgery in medically fit patients who wish to become catheter free.
Reports describing a bladder wrap procedure in patients with DU began to emerge in the late 1990’s. The technique involves harvesting the lattissmus dorsi muscle, the pedicle is then anastomosed to the inferior epigastric vessels and nerve coapted to the intercostal nerve. The muscle is wrapped around the bladder and anchored to the pelvic floor. Long-term follow-up of a series of 24 catheter dependent patients showed that 17 were able to void (mean PVR 25ml) [24]. Significant complications occurred in a third of patients (e.g. venous thromboembolism and pelvic abscess) and consequently at present this approach remains experimental.
Conclusion
DU is a poorly understood and under-researched bladder dysfunction. The symptom based correlate of DU is UAB, however this term remains unclearly defined. There is a need for further epidemiological studies to better understand the population prevalence of DU / UAB and identify at risk groups. The aetiology of DU is multifactorial whilst the pathophysiological mechanisms are incompletely elucidated in many cases. In future there is a need to develop a better understanding of the mechanisms involved in the generation of normal detrusor contraction to facilitate the development of effective treatments, which are at present drastically lacking.
References
1. Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourology and Urodynamics 2002;21:167-78.
2. Chapple CR, Osman NI, Birder L, et al. The Underactive Bladder: A New Clinical Concept? European Urology 2015;68:351-3.
3. Osman NI, Chapple CR, Abrams P, et al. Detrusor underactivity and the underactive bladder: a new clinical entity? A review of current terminology, definitions, epidemiology, aetiology, and diagnosis. European Urology 2014;65:389-98.
4. Resnick NM, Yalla SV, Laurino E. The pathophysiology of urinary incontinence among institutionalized elderly persons. The New England Journal of Medicine 1989;320:1-7.
5. Elbadawi A, Yalla SV, Resnick NM. Structural basis of geriatric voiding dysfunction. II. Aging detrusor: normal versus impaired contractility. The Journal of Urology 1993;150:1657-67.
6. Feber JL, van Asselt E, van Mastrigt R. Neurophysiological modeling of voiding in rats: urethral nerve response to urethral pressure and flow. The American Journal of Physiology 1998;274:R1473-81.
7. Bump RC. The urethrodetrusor facilitative reflex in women: results of urethral perfusion studies. American Journal Of Obstetrics and Gynecology. 2000;182:794-802.
8. Suskind AM, Smith PP. A new look at detrusor underactivity: impaired contractility versus afferent dysfunction. Current Urology Reports 2009;10:347-51.
9. Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: minimum 10-year urodynamic follow-up of untreated bladder outlet obstruction. BJU International 2005;96:1301-6.
10. Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. British Journal of Urology 1979;51:129-34.
11. Schafer W, Langen P-H, et al. A simplified graphic procedure for detailed analysis of detrusor and outlet function during voiding. Neurourology and Urodynamics 1989;8:405-7.
12. Rosario DJ, Woo HH, Chapple CR. Definition of normality of pressure-flow parameters based on observations in asymptomatic men. Neurourology and Urodynamics 2008;27:388-94.
13. Schmidt F, Shin P, Jorgensen TM, et al. Urodynamic patterns of normal male micturition: influence of water consumption on urine production and detrusor function. The Journal of Urology 2002;168:1458-63.
14. Pfisterer MH, Griffiths DJ, Schaefer W, Resnick NM. The effect of age on lower urinary tract function: a study in women. Journal of the American Geriatrics Society 2006;54:405-12.
15. Griffiths DJ. The mechanics of the urethra and of micturition. British Journal of Urology 1973;45:497-507.
16. Rosario DJ, Chapple CR, Tophill PR, Woo HH. Urodynamic assessment of the bashful bladder. The Journal of Urology 2000;163:215-20.
17. van Koeveringe GA, Rahnama'i MS, Berghmans BC. The additional value of ambulatory urodynamic measurements compared with conventional urodynamic measurements. BJU International 2010;105:508-13.
18. Barendrecht MM, Oelke M, Laguna MP, Michel MC. Is the use of parasympathomimetics for treating an underactive urinary bladder evidence-based? BJU International 2007;99:749-52.
19. Katona F, Berenyi M. [Intravesical transurethral electrotherapy of bladder paralysis]. Orvosi Hetilap 1975;116:854-6.
20. Thomas AW, Cannon A, Bartlett E, et al. The natural history of lower urinary tract dysfunction in men: the influence of detrusor underactivity on the outcome after transurethral resection of the prostate with a minimum 10-year urodynamic follow-up. BJU International 2004;93:745-50.
21. Javle P, Jenkins SA, Machin DG, Parsons KF. Grading of benign prostatic obstruction can predict the outcome of transurethral prostatectomy. The Journal of Urology 1998;160:1713-17.
22. Rollema HJ, Van Mastrigt R. Improved indication and follow-up in transurethral resection of the prostate using the computer program CLIM: a prospective study. The Journal of Urology 1992;148:111-15.
23. Monoski MA, Gonzalez RR, Sandhu JS, et al. Urodynamic predictors of outcomes with photoselective laser vaporization prostatectomy in patients with benign prostatic hyperplasia and preoperative retention. Urology 2006;68:312-17.
24. Gakis G, Ninkovic M, van Koeveringe GA, et al. Functional detrusor myoplasty for bladder acontractility: long-term results. The Journal of Urology 2011;185:593-9.
25. Chapple C, Osman N. The Underactive Detrusor. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA (Eds). Campbell-Walsh Urology 11th Edition Elsevier; 2015.

* International Continence Society
www.ics.org
Declaration of competing interests: The author has received speakers’ honoraria and an educational grant from Astellas.