The incidence of renal cell carcinoma (RCC) in the UK has increased steadily over the last two decades, largely driven by the increasing use of abdominal imaging and the incidental detection of small renal lesions [1]. The majority of incidental lesions are benign cysts and whilst these can largely be characterised on the initial imaging examination, some lesions remain indeterminate.
Approximately 40% of patients have at least one renal cyst incidentally discovered on abdominal CT and the prevalence increases with age, from <10% under 40 years to >60% over 80 years [2]. The differential diagnosis for the indeterminate renal lesion is broad and largely includes hyperdense cysts, complex epithelial cysts, RCC, metastases, oncytomas and fat poor angiomyolipomata (AML).
Studies suggest that up to 20% of surgically removed lesions less than 4cm in diameter are benign [3]. A more recent systematic review of 19 studies investigated benignity based on lesion size and revealed that 40% of excised lesions <1cm were in fact benign [4]. In several surgical series, oncocytoma accounted for approximately 50% of all small non-RCC resected lesions [3,4,5]. Biopsy is therefore very useful in obtaining tissue diagnosis and preventing unnecessary intervention.
In general, the management options for the small incidental renal lesion are discussed at a multidisciplinary setting and include leaving the lesion alone (for instance in the elderly, co-morbid or palliative patient), further imaging characterisation, percutaneous biopsy, active surveillance, percutaneous ablation and nephron sparing surgery.
The role of ultrasound
Ultrasound is a quick and inexpensive method for characterising small indeterminate lesions identified on portal venous phase CT, for example the < 2 cm lesion with attenuation value between 20-40HU likely to represent a hyperattenuating cyst. Focused ultrasound is particularly useful for the elderly co-morbid patient who cannot undergo contrast CT due to renal impairment or for a patient who is unable to tolerate an MRI scan. If a given lesion is well-circumscribed, anechoic and avascular, with posterior acoustic enhancement, it can be safely dismissed as a benign cyst. However, care must be taken since papillary RCC can often demonstrate low internal echoes, therefore appearing quite hypoechoic on ultrasound.
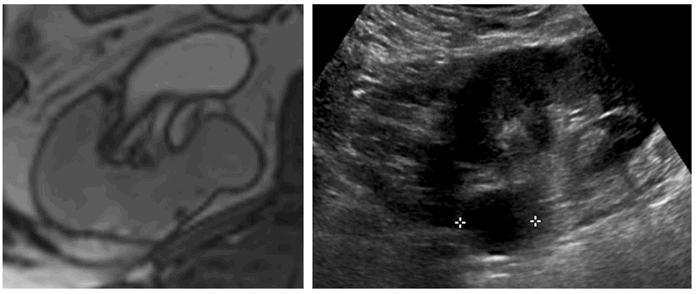
Figure 1: Incidental right indeterminate renal lesion on T2 weighted prostate MRI (left).
Subsequent focused ultrasound demonstrates a well circumscribed anechoic lesion consistent with a benign cyst (right).
On ultrasound, solid renal masses are conspicuous only when their echogenicity is different from the adjacent renal parenchyma, there is distortion of normal renal contour or there is abnormal vascularity on colour doppler (Figure 2).
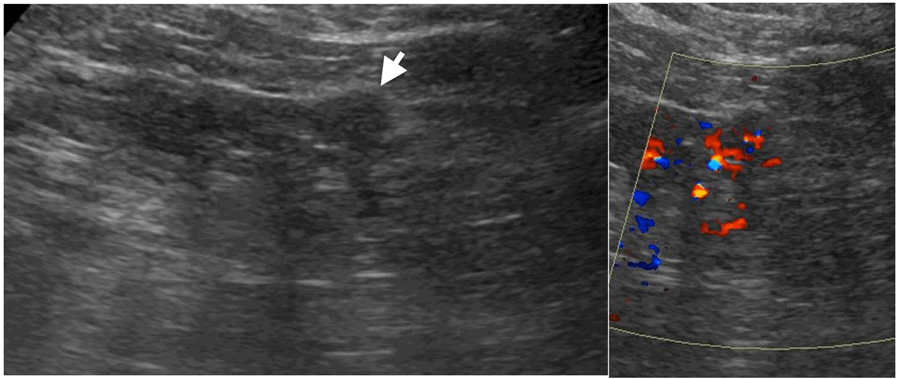
Figure 2: Small partly exophytic RCC on ultrasound (left).
Internal vascularity demonstrated on colour Doppler (right).
Whilst highly echogenic lesions are likely to represent AMLs, they cannot be entirely differentiated from RCCs on ultrasound; therefore it is essential to confirm the presence of macroscopic fat on unenhanced CT or MRI. Following the initial characterisation study, < 2 cm lesions can be followed up by ultrasound alone.
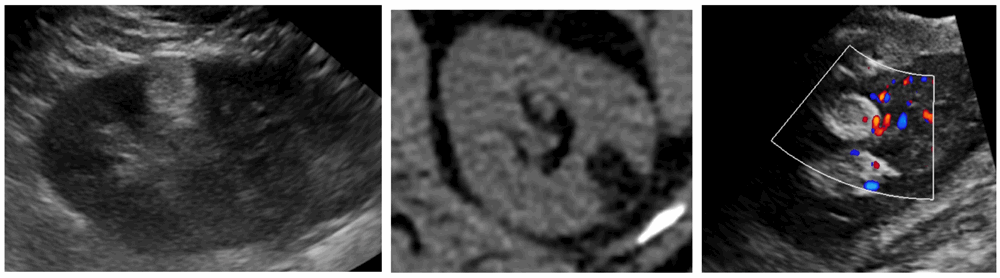
Figure 3: Hyperechoic 1.8cm interpolar lesion on ultrasound (left).
Unenhanced CT demonstrates macroscopic fat (-85 HU, centre).
Follow-up ultrasound at 12 months demonstrates no interval growth (right).
Contrast enhanced ultrasound (CEUS) with microbubble injection is an emerging tool in small renal mass characterisation and serves to identify abnormal vascularity. Although CEUS cannot differentiate malignant from benign renal masses, for instance RCC from oncocytoma, CEUS can reliably differentiate simple cystic lesions from solid vascularised lesions (Figure 4) [6]. CEUS is particularly useful for assessing septal vascularity within complex cystic lesions and problem solving in cases where CT / MRI assessment of small renal lesions proves indeterminate.
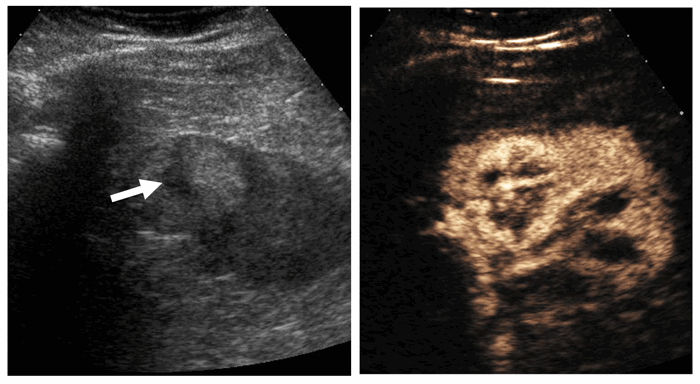
Figure 4: Mildly hyperechoic renal lesion on US (left).
Avid enhancement following microbubble injection (right).
Courtesy of Dr Cherian George, UHNM NHS Trust.
CT characterisation of the small renal mass
Multi-phase CT is the gold standard for characterisation of the small renal mass. In order to evaluate contrast enhancement, unenhanced image acquisition should be followed by a nephrographic phase study (90-120s delay) [7]. The nephrographic phase is the optimum phase to characterise renal lesions since there is maximal and homogeneous enhancement of the normal renal parenchyma; this therefore allows the detection of renal masses which enhance differently.
An increase in attenuation (or enhancement gradient) of a lesion between unenhanced and nephrographic phase indicates the presence of solid vascularised tissue. It is however recognised that cysts can artefactually demonstrate pseudoenhancement (up to 10HU), the degree of which is influenced by factors such as cyst size, location related to renal parenchyma, beam hardening artefact and errors in image reconstruction algorithms [8]. A renal lesion that demonstrates an enhancement gradient <10HU is therefore considered insignificant, between 10-20HU is considered indeterminate and a gradient ≥20 HU is considered to represent definitive contrast enhancement consistent with RCC (Figure 5) [7].
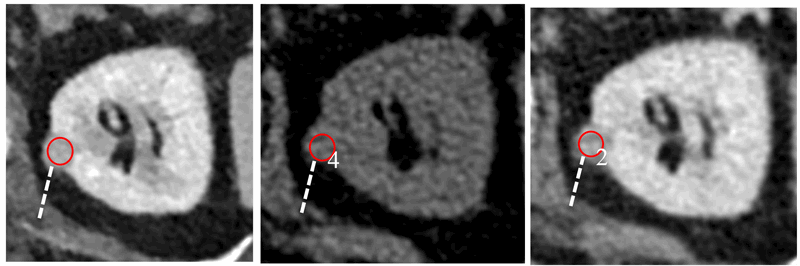
Figure 5: Portal venous phase CT in a 42-year-old male with abdominal pain reveals an incidental 1.2cm left renal lesion (40HU, left). Subsequent multiphase renal CT demonstrates enhancement gradient of 24HU, consistent with a primary renal tumour (centre and right). Excision revealed a type I papillary RCC.
Distinct CT imaging features can allow differentiation of the various histological RCC subtypes. For instance, clear cell RCC typically appears more heterogenous and hypervascular, with a greater tendency for cystic change and tumour necrosis [9]. Papillary RCC (PRCC) appears more hyperattenuating relative to renal parenchyma on unenhanced CT. PRCC also appears more homogenous with relatively reduced vascularity, therefore demonstrating lower enhancement gradients, more likely to fall within the indeterminate range (Figure 6) [9].
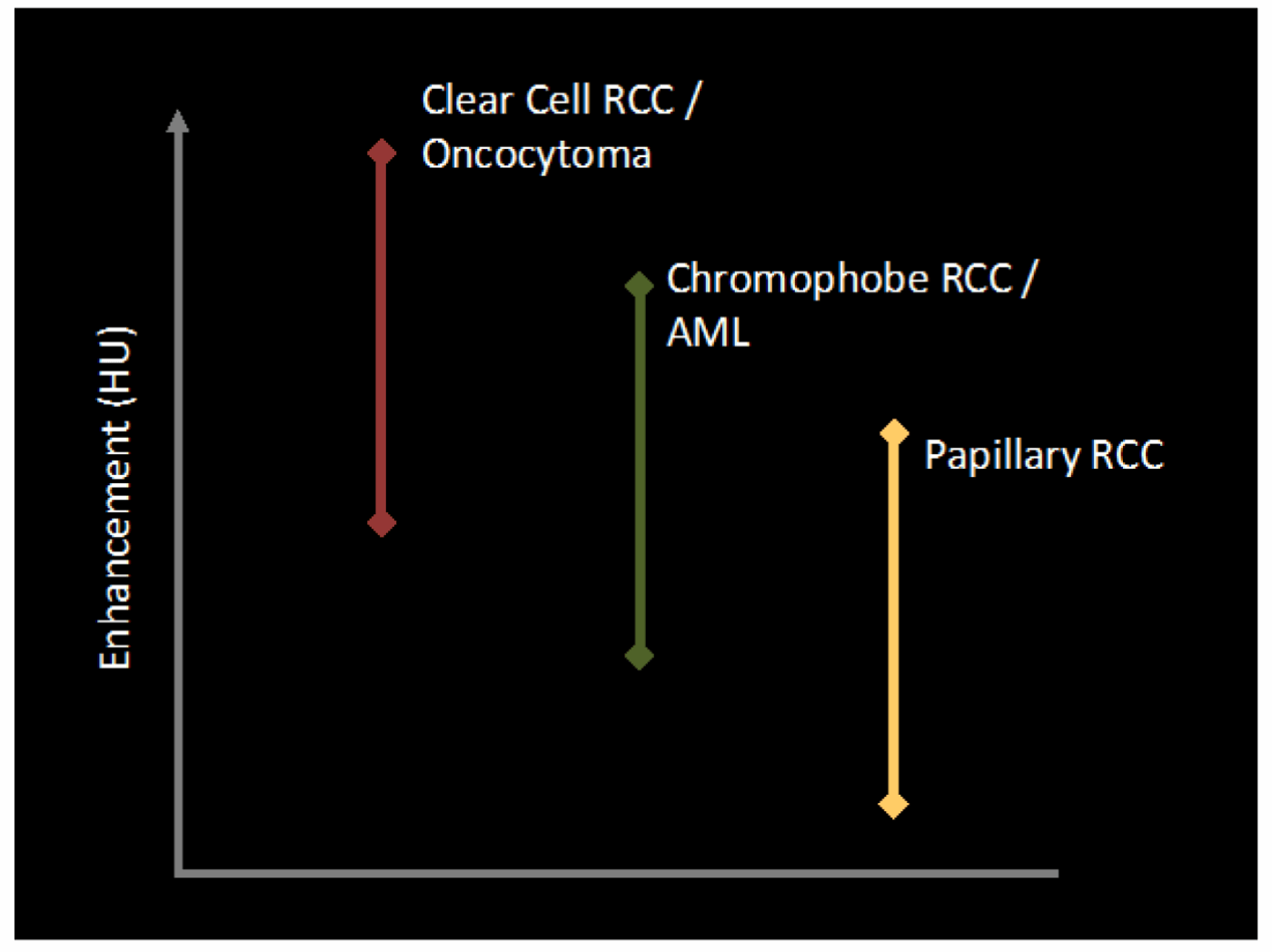
Figure 6: Enhancement characteristics of individual tumour subtypes.
Papillary RCC enhances the least [11].
In view of this heterogeneity of imaging appearances, the size and placement of the ‘region of interest’ (ROI) circle within a given renal lesion may influence enhancement gradient measurement and therefore the ability to reliably diagnose RCC. A recent study revealed smaller ROI circles (~0.5cm2) placed peripherally within a renal lesion had a higher accuracy in differentiating RCC and papillary RCC from cysts [10].
Benign lesions such as oncocytomas and AMLs of course also demonstrate variable contrast enhancement due to their inherent vascularity. The classic central stellate scar in oncocytoma is only seen in a small proportion of cases and oncocytoma cannot be reliably distinguished from RCC on imaging alone. Macroscopic fat within a renal lesion is diagnostic of an AML, however approximately 5% of AMLs do not demonstrate macroscopic fat on CT or MRI, otherwise known as fat poor AMLs. In the presence of calcification, RCC can often demonstrate macroscopic fat due to metaplastic bone formation, however without calcification, macroscopic fat within RCC is extremely rare (Figure 7).[12].
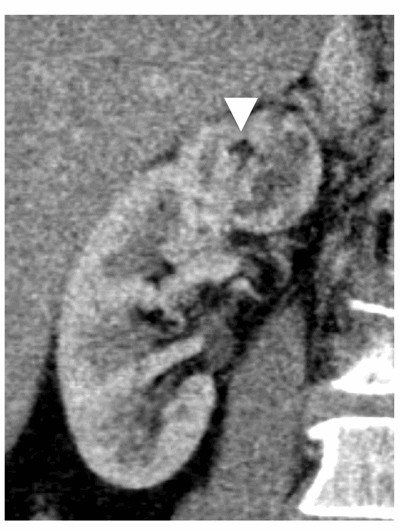
Figure 7: Chromophobe RCC containing a small island of macroscopic fat.
Hyperdense cysts contain colloid or haemorrhagic material and therefore appear ‘bright’ on pre-contrast imaging, with attenuation values characteristically >70HU. Hyperdense cysts should typically measure <3cm in diameter, appearing homogenous, round and thin-walled, with enhancement gradients <10HU (Figure 8) [13].
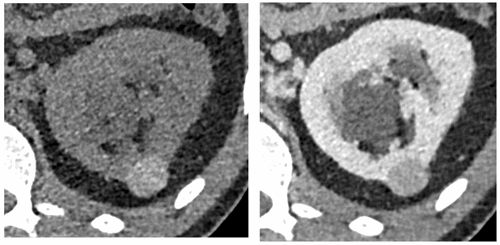
Figure 8: Hyperdense cyst. Attenuation value 75HU on unenhanced CT (left).
No significant contrast enhancement on nephrographic phase, 80 HU (right).
Due to the variability in the composition of cyst contents, attenuation values of hyperattenuating cysts on CT can be less than 70HU; hence without an unenhanced phase to measure contrast enhancement it is difficult to differentiate hyperattenuating cysts from solid enhancing lesions on a single portal venous phase examination (such as that performed for conventional abdominal CT). Incidental lesions with soft tissue attenuation on portal venous phase CT (20-100) therefore require careful comparison with previous available imaging or further characterisation with focused ultrasound, CT or MRI (Figure 9).
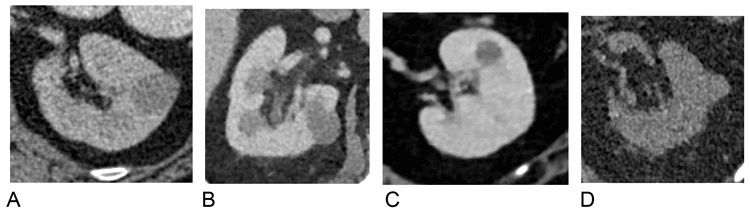
Figure 9:
A) Hyperattenuating cyst identified on lung cancer staging CT (42HU), portal venous phase. No enhancement on subsequent multi-phase CT.
B) Hyperattenuating cyst identified on lymphoma staging CT (38HU), portal venous phase.
C) Hyperattenuating lesion identified on CT Colonogram (42HU), portal venous phase. Further MRI characterisation revealed a benign cyst.
D) Hyperattenuating cyst identified on CT KUB for renal colic (38HU), unenhanced phase.
The role of MRI
MRI is often very useful when a) renal CT characterisation is equivocal, b) the patient cannot undergo CT due to renal impairment or iodine contrast allergy, c) in young patients where it is important to limit radiation exposure, and d) for very small tumours (<1cm) where MRI may prove to be more diagnostic. Like CEUS, MRI is particularly useful in assessing septal thickening and enhancement in cystic renal lesions; this is of importance when there is uncertainty in differentiating Bosniak IIF from Bosniak III lesions on CT, both of which would have different clinical management.
Papillary RCC is often hypointense on T2-weighted sequences and demonstrates low-level homogenous contrast enhancement (Figure 10). Clear cell RCC on the other hand appears hyperintense on T2-weighted sequences with intense heterogenous contrast enhancement and early wash-out.
Oncocytoma demonstrates variable MRI signal characteristics and cannot reliably be differentiated from RCC. Fat suppressed images and signal loss on opposing phase sequences will identify fat within a classic AML. Signal loss on opposing phase sequences however cannot reliably differentiate fat poor AML from RCC since clear cell RCC also contains small amounts of intracellular fat. The presence of ‘India ink artefact’ at the interface between the lesion and renal cortex (appearing as a thick black line) more reliably differentiates a fat poor AML from RCC [14].
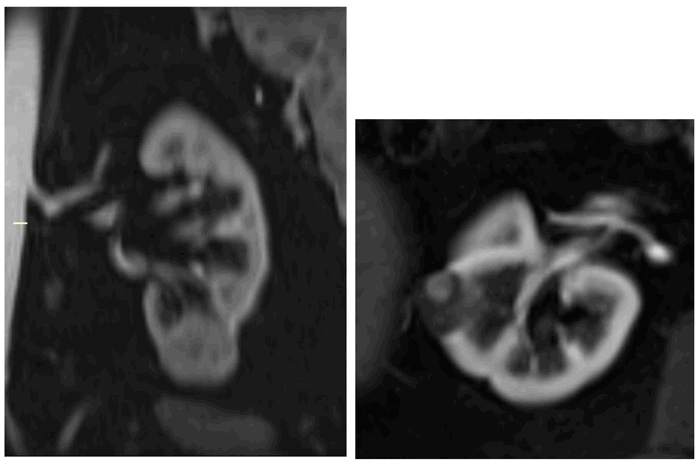
Figure 10: Type I papillary RCC demonstrating quite low-level homogenous enhancement (left).
Bosniak IV cystic lesion within the contralateral kidney (right) also excised revealing papillary RCC.
The role of percutaneous biopsy
Ultrasound or CT guided percutaneous biopsy is a safe and effective procedure with a negligible risk of tumour seeding [15]. Biopsy is particularly helpful for assessing the indeterminate renal lesion on CT or MRI, particularly where clinical management would be strongly influenced by specific pathology.
With the emerging use of thermal ablative techniques (largely cryoablation and radiofrequency ablation), it is essential to perform percutaneous biopsy prior to ablation in order to prevent the unnecessary treatment of histologically benign lesions. Modern immunocytochemistry techniques are now able to reliably differentiate oncocytoma from RCC. In view of the fact that up to 20% of small renal masses are benign and most often oncocytomas, biopsy could also avoid unnecessary surgical intervention.
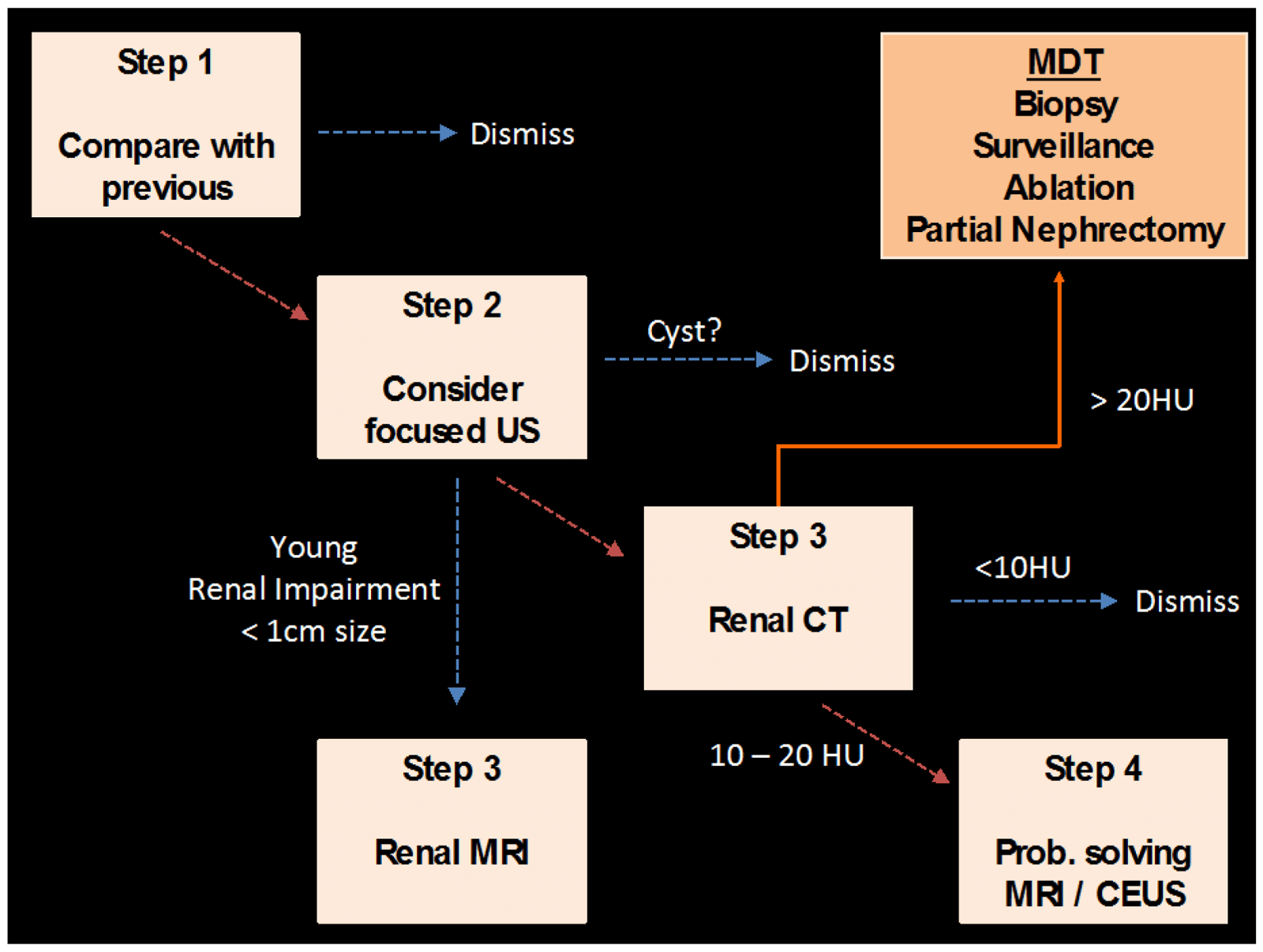
Figure 11: Proposed algorithm for assessment of the indeterminate renal lesion
detected on conventional abdominal CT (portal venous phase).
References
1. Hollingsworth JM, Miller DC, Duignalt S, et al. Rising Incidence of Small Renal Masses: a Need to Reassess Treatment Effect. J Nat Cancer Inst 2006;98:1331-4.
2. Carrim ZI, Murchison JT. The prevalence of simple renal and hepatic cysts detected by spiral computed tomography. Clin Radiol 2003;58:626-9.
3. Duchene DA, Lotan Y, Cadeddu JA, et al. Histopathology of surgically managed renal tumors: Analysis of a contemporary series. J Urol 2003;62(5):827-30.
4. Johnson DC, Vukina J, Smith AB, et al. Preoperatively misclassified, surgically removed benign renal masses: a systematic review of surgical series and United States population level burden estimate. J Urol 2015;193(1):30-5.
5. Silver DA, Morash C, Brenner P, et al. Pathologic findings at the time of nephrectomy for renal mass. Ann Surg Oncol 1997;4:570-4.
6. Tamai H, Takiguchi Y, Oka M. Contrast-enhanced ultrasonography in the diagnosis of solid renal tumors. J Ultrasound Med 2005;24(12):1635-40.
7. Berland LL, Silverman SG, Gore RM, et al. Managing incidental findings on abdominal CT: white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2010;7:754-73.
8. Birnbaum BA, Hindman N, Lee J, et al. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology 2007;244:767-75.
9. Kim JK, Kim TK, Ahn HJ, et al. Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 2002;178:1499-506.
10. Rosenkrantz AB, Matza BW. Impact of size of region-of-interest on differentiation of renal cell carcinoma and renal cysts on multi-phase CT: preliminary findings. Eur J Radiol 2014;83(2):239-44.
11. Zhang J, Lefkowitz RA, Ishill NM, et al. Solid renal cortical tumors: differentiation with CT. Radiology 2007;244(2):494-504.
12. D’Angelo PC, Gash JR, Horn AW, Klein FA. Fat in renal cell carcinoma that lacks associated calcifications. AJR 2002;178:931-2.
13. Bosniak MA. Difficulties in classifying cystic lesions of the kidney. Urol Radiol 1991;13:91-3.
14. Halpenny D, Snow A, McNeill G, Torreggiani WC. The radiological diagnosis and treatment of renal angiomyolipoma - current status. Clin Radiol 2010;65:99-108.
15. Silverman SG, Gan YU, Mortele KJ, et al. Renal masses in the adult patient: the role of percutaneous biopsy. Radiology 2006;240:6-22.
“Multi-phase CT is the gold standard for characterisation of the small renal mass.”
TAKE HOME MESSAGE
-
The exponential growth of cross-sectional imaging has significantly contributed to a similar rise in the detection of incidental renal lesions.
-
Small indeterminate renal lesions on conventional abdominal CT (portal venous phase) should be carefully compared to previous available imaging or undergo further imaging characterisation with focused ultrasound, multi-phase renal CT or contrast-enhanced MRI as proposed in Figure 11.
-
On multi-phase CT, a contrast enhancement gradient of ≥20HU signifies solid vascularised tissue compatible with a primary renal lesion. Benign lesions also demonstrate variable contrast enhancement and represent up to 20% of resected lesions <4cm.
-
Indeterminate lesions on CT (enhancement gradient 10-20HU) should undergo further imaging assessment with CEUS or MRI.
-
Percutaneous biopsy should be considered for lesions remaining indeterminate after all imaging modalities have been exhausted. Biopsy prior to percutaneous ablation is essential in order to prevent unnecessary treatment of histologically benign lesions.
Declaration of competing interests: None declared.
Acknowledgement: Miss Charlotte Foley, Consultant Urologist, Lister Hospital, Stevenage.



