The incidence and prevalence of kidney stones is increasing [1,2]. Significant recurrence rates are noted with 14% of patients experiencing a further episode at one year, 35% at five years, and 52% at 10 years [3]. Over 10% of stone formers experience multi-recurrent disease [4]. Urolithiasis represents a worsening public health issue: the financial burden of it in the United States in 2005 was estimated to be greater than $2 billion [5].
A simple metabolic screen should be routine practice in all individuals who present with stone disease, with detailed analysis reserved for those more likely to have recurrence. As well as helping to prevent recurrences and their sequelae, metabolic screening may reveal an underlying metabolic disorder or other disease process, which can then be managed appropriately.
The aim of this article is to provide the general urologist with a rounded knowledge of the metabolic aspects of urolithiasis. We will discuss what a basic and detailed metabolic stone screen involves and will consider which patients should have which. We will suggest how and when this should be done and how the results are to be interpreted. Finally, we will review specific metabolic conditions implicated in recurrent urolithiasis and examine treatment options for each. The boxes contain information the authors feel may be of particular use to candidates approaching their FRCS (Urol) MCQs and Vivas.
Who to test
The European Association of Urology (EAU) guidelines recommend that all patients who present to hospital – be it in an emergency or elective setting – with a renal tract calculus should have a basic metabolic screen performed [6]. This includes a urine dipstick to assess for the presence of blood, leucocytes and nitrites and to measure urinary pH. Urine should also be sent for MC&S. Blood tests should be sent to evaluate the serum creatinine, sodium and potassium, ionised calcium and uric acid. Whenever possible, any calculus collected by the patient or retrieved at the time of surgery should be sent for biochemical analysis.
‘High risk’ patients should undergo a more detailed analysis, involving gathering of a 24-hour urine collection, as detailed below. The term ‘high risk’ may relate to patients who either have an increased likelihood of recurrence, or those in whom a recurrence will be of greater consequence. Factors that predispose to an increased likelihood of recurrence include having formed multiple stones in the past or having formed bilateral stones, especially if the intervals between recurrences have been short.
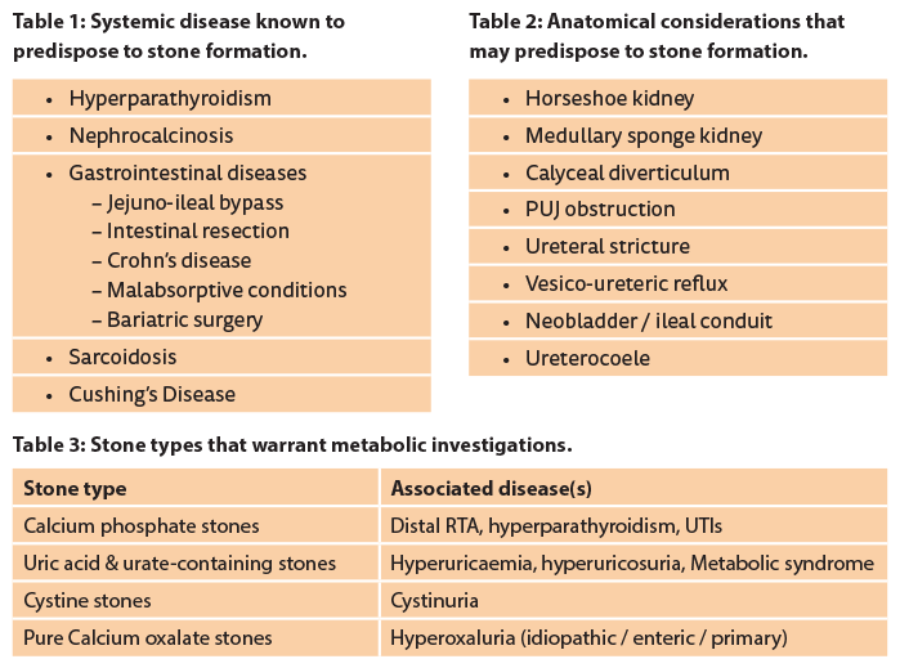
Some patients who have only had a single episode of stones may still be at high risk of recurrence and therefore warrant a detailed metabolic evaluation. These include those who presented at a young age, with 25 years of age being one suggested cut-off for significance, those in whom there is a strong family history of stone formation, those in whom residual fragments remain after urological intervention, those who have a particular disease known to predispose to stone formation (Table 1), those who have anatomical considerations that may predispose to stone formation (Table 2) and those who form either ‘pure stones’ or stone-types that are typically associated with underlying metabolic abnormalities (Table 3).
How to test
Stones should be sent for analysis at the time of passage / retrieval. Further stone analysis should be conducted if patients have recurrence despite appropriate pharmacological therapy, early recurrence despite being stone free or late recurrence after a long stone-free period as the stone composition may change.
A detailed clinical history should be taken to elicit risk factors for stone formation in the past medical history (Tables 1 & 2), particularly noting the extent of previous stone disease, what treatment was required, and any family history. The patient’s occupation, history of heat exposure, typical fluid intake, and dietary habits should be documented, as should their weight and body mass index.
Plasma biochemistry is checked, specifically assessing the serum creatinine, electrolytes, calcium, phosphate, bicarbonate, uric acid, parathyroid hormone and vitamin D (if calcium high).
As well as the bedside urine tests mentioned above, a urine dipstick performed in the context of a metabolic work up should note the specific gravity of the urine and the presence of proteinuria. A spot cystine screen may also be performed (see overleaf).
There is limited evidence to suggest when is the most appropriate time to perform a 24-hr urine analysis, but most authorities agree that it should be done once the patient is stone free; an interval of 20 days after achieving this is suggested [7,8] and has been endorsed by EAU guidelines [6].
Once a patient has commenced pharmacological intervention for a metabolic condition repeat analysis should be considered to allow drug dosages to be adjusted. Patients should then be followed up at 12 months according to perceived risk and in relation to other underlying conditions.
Spot cystine screen
(Also known as the cyanide nitroprusside test or Brand’s test)
-
Add sodium cyanide to urine, let it stand for 10 minutes
– Disulphide bonds broken by cyanide liberating cysteine from cystine and homocysteine from homocystine. -
Sodium nitroprusside is added
– A positive test result (cysteine / homocysteine present) is confirmed if the solution turns a red / purple colour. -
If positive 24-hour urine collection should be undertaken to confirm and quantify levels of urinary cystine / homocystine.
How to collect a 24-hour urine
Two collections on consecutive days. Intake and activity should be typical for patient. Start in morning, making a note of the time. Void / empty bladder and discard. All voids for next 24hrs, including first void the next morning, collected in specialised containers. Stored in a cool place. Take to pathology department of hospital promptly.
-
Hydrochloric acid
– Used as a preservative to prevent precipitation of calcium oxalate and calcium phosphate crystals and prevent oxidation of ascorbate to oxalate. Used to measure volume, creatinine, calcium, oxalate, citrate, magnesium and phosphate. -
Sodium azide
– Prevents precipitation of uric acid. Used to measure volume, creatinine, pH, urate, protein, cystine / homocystine, sodium and potassium.
General advice for all stone formers
All patients who form a urinary tract calculus should be encouraged to maintain a healthy weight (BMI 18-25 kg/m2), to undertake adequate exercise and have a healthy and balanced diet, rich in vegetables and fibre, with normal calcium content (1-1.2 g/day), limited NaCl content: (4-5g/day) and limited animal protein content (0.8-1.0g/kg/day) [9].
An inverse relationship between high fluid intake and stone formation has been repeatedly demonstrated and patients should be advised to drink 2.5 to 3L per day, achieved by regular intake, to maintain a urine output between 2 and 2.5L per day. For those who are well motivated or who already use urine dipsticks to monitor their pH, a specific weight of urine < 1010 should be targeted [10,11].
Fresh fruit juice contains citrate, a urinary inhibitor, and bicarbonate, which helps to alkalinise urine. If potassium is present, both pH and citrate are increased also [12,13].
Specific metabolic conditions
Hypercalciuria (urine calcium excretion >7.5mmol/24h in men; >6.1mmol/24h in women [14]), in the presence of a normal serum calcium, is usually idiopathic and may be due to an absorptive phenomenon (patients absorb and excrete an elevated proportion of their dietary calcium intake), or reduced renal calcium reabsorption, also known as renal leak. Subtle differences in phosphate levels (raised in absorptive, low in renal), parathyroid hormone levels (suppressed in absorptive, raised [secondarily] in renal) and fasting urinary calcium (normal in absorptive, remains elevated in renal) help to differentiate between these causes.
In rare cases hypercalciuria may result from certain tubulopathies (dRTA, Dent’s, some Fanconi’s, Bartter’s), some malignancies (lymphoma, leukaemia, multiple myeloma), sarcoidosis, Paget’s disease and certain drugs (e.g. furosemide, topiramate). In these cases, referral and management by an appropriate specialist is recommended.
Both absorptive and resorptive (renal leak) hypercalciuria may be successfully treated with thiazide diuretics. Thiazides treat idiopathic hypercalciuria by preventing sodium being exchanged for calcium in the distal convoluted tubule, so that more sodium is excreted in the urine and correspondingly less calcium. In renal hypercalciuria this corrects the underlying problem, but with absorptive hypercalciuria the benefits may be short lived and a ‘thiazide holiday’ may be beneficial. Hypokalaemia may occur as a result of thiazide therapy, which may induce cellular acidosis and hypocitraturia, and should be tested for and treated with potassium supplementation, as required [15]. Potassium citrate is ideal for management of this effect.
The most common cause of hypercalciuria with an elevated serum calcium is hyperparathyroidism. This causes a resorptive hypercalciuria with increased gut absorption and increased bone resorption of calcium. This can be identified by measuring parathyroid hormone. Eighty percent of cases of primary hyperparathyroidism are caused by a benign parathyroid adenoma. Parathyroid carcinomas are rarely the cause, but prompt referral for a specialist assessment is mandatory. Surgical parathyroidectomy usually corrects hyperparathyroidism.
Renal tubular acidosis describes a group of conditions in which the failure of the kidneys to excrete acid into the urine results in an inappropriately alkaline urine and a metabolic acidosis.
Type 1, also known as distal renal tubular acidosis (dRTA), is associated with a failure of the cells of the distal nephron to excrete hydrogen ions into the urine. The resulting acidaemia is accompanied by hypokalaemia as potassium ions cannot be reabsorbed. The metabolic acidosis also propagates loss of calcium into urine and the alkaline urine reduces tubular reabsorption of citrate causing hypocitraturia. The result is nephrocalcinosis and renal tract calculi, most commonly calcium phosphate stones.
Types 2, 3 and 4 RTA do not cause urinary tract calculi.
The diagnosis of RTA is confirmed by the presence of a metabolic acidosis with alkaline urine in the presence of a normal glomerular filtration rate (GFR). The furosemide and fludrocortisone (F&F) test involves the simultaneous administration of oral furosemide and fludrocortisone. If the patient adequately acidifies their urine (i.e. reaches a nadir pH ≤5.4), they do not have an acidification defect. If not, or if the results are equivocal, they will be assessed with a formal acidification test. The ammonium chloride urinary acidification test, whereby the patient is loaded with ammonium chloride, stresses the ability of the kidneys to excrete acid and helps to identify the RTA subtype. It is a potentially dangerous test and often causes vomiting. These tests and the ensuing management are usually carried out by a renal physician or biochemist. The principal of treatment of distal RTA is to address the metabolic acidosis with alkali therapy, such as sodium bicarbonate or potassium citrate. The latter also helps to correct potassium levels and remedies the hypocitraturia.
Hyperoxaluria, defined as oxalate excretion > 40mg/24 hrs can be primary enteric, or idiopathic. Mildly elevated levels of urinary oxalate (40-80mg/24 hrs) may be caused by increased consumption of exogenous oxalate. Foods containing high levels of oxalate include chocolate, nuts, strawberries and leafy vegetation such as rhubarb, spinach and tea; these should be moderated in patients with hyperoxaluria.
At physiological pH, such as in the gut, oxalate has a high affinity for calcium and forms an insoluble salt, which is not absorbed. Only remaining free oxalate is absorbed, and absorption of oxalate is therefore inversely proportional to calcium intake, hence the finding that a low calcium diet has been shown to increase oxalate absorption and its renal excretion.
Enteric hyperoxaluria results from gastrointestinal disorders associated with increased absorption of dietary oxalate. Examples include inflammatory bowel diseases, post ileal resection, chronic diarrhoea and other malabsorptive conditions. Recently the putative intestinal anion exchange transporter Slc26a6 has been shown to be involved in intestinal oxalate transport and may be an important therapeutic target in the future [16,17]. Furthermore, the gram negative obligate anaerobe Oxalobacter Formigenes degrades oxalate and has been proposed as a mechanism of reducing oxalate absorption [18]. A recent study has shown there to be a lower prevalence of Oxalobacter Formigenes in recurrent calcium oxalate stone formers, supporting this theory [19]. Intestinal colonisation may be affected by antibiotic use, perhaps making patients with recurrent urinary tract infections more prone to hyperoxaluria and oxalate stones.
Endogenous oxalate is an end product of hepatic metabolism of glycolate. Primary hyperoxaluria is a collection of genetic disorders which result in excess production of endogenous oxalate. There are three types, but specific genetic mutations have only been identified in types 1 and 2 (see below).
Primary hyperoxaluria:
Type I – the most common form. Caused by an autosomal recessive mutation in the gene encoding hepatic alanine-glyoxylate aminotransferase (AGXT). It causes glycolic aciduria and hyperoxaluria.
Type II – caused by a mutation in the gene encoding hepatic glyoxylate reductase / hydroxypyruvate reductase (GRHPR). It causes L-glyceric aciduria and hyperoxaluria.
Type III – no specific enzyme deficiency has been identified. It may be due to inborn error of oxalate metabolism.
General steps in the management of hyperoxaluria include maintaining good fluid intake, reducing intake of oxalate-rich foods, reducing fat, sodium and animal protein, taking calcium supplements at meal times to encourage calcium-oxalate complex formation in the intestine and using alkaline citrate to raise urinary pH and correct hypocitraturia.
Primary hyperoxaluria should be managed by an experienced multidisciplinary team in a specialist centre. These patients are required to maintain a fluid intake of 3.5 to 4L per day, use an alkaline citrate and may be treated with pyridoxine to reduce urinary oxalate excretion (effective in about 1/3 patients). Untreated primary hyperoxaluria can result in significant damage to the kidneys and end stage renal failure. The inability of the kidneys to excrete oxalate causes systemic oxalosis with significant consequences.
Cystinuria is a rare, autosomal recessive disease, which is responsible for approximately 1% of renal tract calculi; it is the commonest of the pure metabolic stone disorders. It is characterised by tubular defects in the dibasic amino acid transporter in the proximal convoluted tubules, which confers an inability to reabsorb the amino acids cystine, lysine, ornithine and arginine from the proximal convoluted tubules of the kidney. Two genes have been commonly identified as implicated in cystinuria: SLC3A1 (chromosome 2) and SCLC7A9 (chromosome 19). Ornithine, lysine and arginine are all highly soluble in urine and therefore do not form stones. The solubility of cystine varies with urine acidity and at lower pH levels significant urolithiasis may occur.
Normal individuals excrete <30mg cystine in their urine per 24 hrs, homozygotes typically excrete >400mg per 24 hrs and heterozygotes somewhere in the middle. Around pH 7 the solubility limit of cystine is around 250mg/L and doubles to around 500mg/L at pH above 7.5 [20]. Therapy is therefore aimed at decreasing concentration below solubility limits by alkalinising the urine. Patients should aim for a urine volume of >3L per 24 hours, which should be distributed evenly throughout the day, ideally by consuming >150 ml/hour during waking hours. Some patients may be advised to wake during the night in order to maintain adequate and continuous urine output. Alkaline citrates, such as potassium citrate are used to maintain a urine pH between 7 and 7.5; alkalising the urine to pH >7.5 puts patients at risk of developing calcium phosphate stones.
Diets low in methionine may reduce the urinary extraction of cystine, but have limited proven efficacy and are unpalatable; compliance is therefore limited. Reducing intake of salt can reduce cystine excretion by 150mg/day and is therefore highly recommended [21]. (Use sodium bicarbonate as an alkalinising agent in these patients with caution, as the increased sodium load may increase cystine excretion.)
Chelating agents are drugs that combine with cystine to form a soluble disulphide complex. D-penicillamine is a first generation chelating agent but is often poorly tolerated due to its significant side-effect profile (rash, arthralgia and GI upset are common, among others). α-mercaptopropionylglycine is a second-generation agent which is more effective and better tolerated than D-penicillamine. Captopril, an ACE-inhibitor is also an effective chelator. Tiopronin (Thiola) undergoes a thiol-disulfide exchange with cystine to form a soluble Tiopronin-Cystine compound [22] and is commonly used in practice today.
Uric acid stones make up 5-10% of all kidney stones.
There is a well-established association between uric acid stone formation, obesity and elevated serum glucose levels / insulin resistance [23,24]. These traits, along with hypertension and elevated cholesterol / triglycerides have been described as the metabolic syndrome, which is becoming an increasing problem in the developed world [25]. As well as uric acid stone formation, the metabolic syndrome predisposes to cardiovascular disease and type 2 diabetes. In other parts of the world metabolic syndrome may be known as Syndrome X or Reaven’s syndrome.
Uric acid is an end product of purine metabolism. Isolated hyperuricosuria (defined as > 4 mmol/day in adults) may result from the consumption of excess dietary animal protein and certain drugs, such as thiazides and salicylates. It may also result from type 2 diabetes mellitus. Hyperuricosuria may be associated with hyperuricaemia in conditions such as gout, lymphoproliferative disorders, Lesch-Nyhan syndrome and certain inborn errors of metabolism.
Uric acid has an ionisation constant (pKa) of 5.5 and below this level uric acid crystals precipitate to form uric acid stones. Acidic urine pH and low urine volume, as well as hyperuricosuria are therefore major risk factors for uric acid stone formation. Acidic urine and low urinary volumes are probably the most important factors as patients with uric acid stones very often have normal levels of urinary uric acid.
Patients should be advised to consume enough fluids such that their urine output is 2 to 2.5L per day. Alterations in diet should include a reduction in the consumption of animal protein, a reduction in sodium intake and a healthy diet and exercise regimen to promote weight loss if required.
Medical therapy is principally aimed at alkalinising the urine. For the purposes of prevention, a urine pH between 6.2 and 6.8 should be sought. Where chemolysis is required a more drastic regimen is needed with a targeted urine pH between 7.0 and 7.2. Alkaline citrates such as potassium citrate and sodium bicarbonate may be used. As well as urinary alkalisation, potassium citrate provides the added benefit of correcting hypocitraturia and thus prevents the growth and aggregation of calcium oxalate crystals and the agglomeration of calcium phosphate crystals. Patients should be taught to monitor their own urine pH using dipsticks at regular intervals and to modify their dosage accordingly. A typical starting regimen of potassium citrate therapy is 10mls, three times per day diluted with water.
Hyperuricosuria, defined as >4.0mmol/day and hyperuricaemia, defined as >380umol/L, can be treated with Allopurinol, a xanthine oxidase inhibitor, (100mg/day for the former group, 100-300mg/day for the latter).
Of note, hyperuricosuria has been shown to be implicated in the formation of calcium oxalate stones, as uric acid crystals serve as a nidus for calcium oxalate crystals to precipitate on. Allopurinol has been shown to be of therapeutic benefit in these patients too.
Summary
Urolithiasis is a significant health issue, both at a public health level and for those patients who experience recurrent episodes, require multiple surgical interventions and suffer the associated long-term health issues that can be associated with it.
Metabolic screening can help to identify underlying metabolic disorders and thereby reduce the frequency and severity of their recurrences. By carefully selecting high risk patients for detailed analysis the burden of work is limited and the cost-benefit ratio, in terms of both time and resources, remains high.
General advice on lifestyle, fluid intake and diet should be issued to all stone formers as they are all at significant risk of recurrence. Patients with an identified metabolic abnormality should be treated accordingly, as per the recommendations of this article. More complex metabolic disease and patients who continue to form stones despite appropriate therapy should be referred to an appropriate specialist for their ongoing care.
References
1. Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol 2010;12(2-3):e86-96.
2. Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 2003;63:1817-23.
3. Uribarri J, Oh MS, Carroll HJ. The first kidney stone. Ann Intern Med 1989;111:1006-9.
4. Keoghane S, Walmsley B, Hodgson D. The natural history of untreated renal tract calculi. BJU Int 2010;105(12):1627-9.
5. Pearle MS, Calhoun EA, Curhan GC. Urologic diseases in America project: urolithiasis. J Urol 2005;173:848-57.
6. Skolarikos A, Straub M, Knoll T, et al. Metabolic evaluation and recurrence prevention for urinary stone patients: EAU guidelines. Eur Urol 2015;67(4):750-63.
7. Norman RW, Bath SS, Robertson WG, et al. When should patients with symptomatic urinary stone disease be evaluated metabolically? J Urol 1984;132(6):1137-9.
8. Hesse AT, Tiselius H-G, Siener R, et al. Urinary stones, diagnosis, treatment and prevention of recurrence. 3rd Edition. Basel, Switzerland: Karger AG; 2009.
9. Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346(2):77-84.
10. Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med 1998;128(7):534-40.
11. Borghi L, Meschi T, Amato F, et al. Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: a 5-year randomized prospective study. J Urol 1996;155(3):839-43.
12. Wabner CL, Pak CY. Effect of orange juice consumption on urinary stone risk factors. J Urol 1993;149(6):1405-8.
13. Gettman MT, Ogan K, Brinkley LJ, et al. Effect of cranberry juice consumption on urinary stone risk factors. J Urol 2005;174(2):590-4.
14. Hodkinson A, Pyrah LN. The urinary excretion of calcium and inorganic phosphate in 344 patients with calcium stone of renal origin. Br J Surg 1958;48:10-8.
15. Semins MH, Matlaga BR. Medical evaluation and management of urolithiasis. Ther Adv Urol 2010;2(1):
3-9.
16. Sakhaee K. Recent advances in the pathophysiology of nephrolithiasis. Kidney Int 2009;75(6):585-95.
17. Danpur C. Primary hyperoxaluria. In: Scriver C, Beaudet A, Sly W, et al. (Eds). The Metabolic and Molecular Basis of Inherited Disease. New York, USA; McGraw-Hill 2001:3323–67.
18. Hatch M, Freel RW. Intestinal transport of an obdurate anion: oxalate. Urol Res 2005;33:1-16.
19. Kaufman DW, Kelly JP, Curhan GC, et al. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol 2008;19:1197-203.
20. Dent CE, Senior B. Studies on the treatment of cystinuria. Br J Urol 1955;27:317-32.
21. Ng CS, Streem SB. Contemporary management of cystinuria. J Endourol 1999;13:647-51.
22. THIOLA [prescribing information]. San Antonio, TX, USA; Mission Pharmacal Company.
23. Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol 2004;172:159-63.
24. Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 2006;17:2026-33.
25. Ford ES, Giles WH, Dietz WH. Prevalence of metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287(3):356-9.
Declaration of competing interests: None declared.






