The UK National Screening Committee has been calling for further research into alternative screening tests for prostate cancer. The committee decided against prostate cancer screening using prostate specific antigen (PSA) testing on the basis that “PSA is still a poor test for prostate cancer and a more specific and sensitive test is needed” [1]. This has triggered a surge of interest into a range of alternatives to PSA for prostate cancer screening.
With the success of MRI as a diagnostic test in secondary care, the question has arisen as to whether MRI could be adapted for widespread use in the general population as a screening test. Whilst there are legitimate concerns over its high cost and limited availability, recent technological advances have meant that a fast (5-10 minute) MRI could be a feasible screening test. Indeed, several clinical trials have commenced assessing such an abbreviated bi-parametric MRI for screening men in the community.
This has received recent national media coverage with journalists writing evocative headlines such as how a “10-minute scan may become the universal screening tool for prostate cancer” [2]. It is not surprising that these headlines have been met with a degree of scepticism by urologists given that we are still in the early phases of accepting MRI in hospitals as a triage before biopsy. This article aims to set out the rationale for MRI screening and provide a balanced perspective behind the headlines.
The case for prostate cancer screening
Prostate cancer screening has been a controversial and widely debated topic for over two decades. Many of the arguments against screening are inextricably linked to screening using PSA. However, if we consider prostate cancer independent of PSA, it has several hallmarks which are suggestive of a condition that could benefit from screening.
The mortality rate from prostate cancer is often under-recognised as the debate tends to focus on the issue of overdiagnosis. The high prevalence of insignificant prostate cancer should not detract from the fact that prostate cancer remains the second most common cause of male death with around 11,700 deaths per year in the UK. Men have a 4.3% lifetime risk of dying from the disease and Afro-Caribbean men have an 8.7% lifetime risk [3]. The disease clearly meets the first criteria for screening as defined by Wilson and Jungner that the condition should be an important health problem.
Prostate cancer is characteristically asymptomatic until a more advanced stage.The disease is often slow-growing and the 10-year survival rate is high even for intermediate and high-risk disease. In the observation arm within the ProtecT trial, 22% of participants had intermediate or high-risk disease and the 10-year prostate cancer related mortality rate was minimal (1.5 deaths per 1000 person years) [4]. This slow progression is an advantage for screening as it should provide a greater window of opportunity for detection and curative treatment provided there is a test which can accurately identify the sub-type disease with a poor prognosis.
The controversy around PSA screening
Most international guidelines have adopted an informed-decision or opportunistic approach to PSA testing in the community. Men can request a PSA test provided the benefits and risks are fully explained, particularly as it is unclear whether the benefits from PSA screening outweigh the harms from false positives and overdiagnosis.
The prolonged natural history of prostate cancer has meant that it has taken over a decade for the results of large randomised controlled screening trials to emerge. The outcomes have been mixed with the three largest trials producing conflicting results. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial and Cluster Randomized Trial of PSA Testing for Prostate Cancer (CAP) did not find any mortality benefit from PSA screening. Both trials were limited with PLCO suffering from contamination within the control arm and CAP evaluating a (low intensity) one-off PSA test strategy. The most robust evidence comes from the European Randomized study of Screening for Prostate Cancer (ERSPC) which evaluated the outcomes of PSA screening across eight European countries. The 16-year outcomes found that PSA can lead to a small improvement in prostate cancer mortality but this was not constant across all countries [5].
Overcoming the problems of overdiagnosis
Although PSA screening may reduce prostate cancer mortality, the major drawback is the high rate of overdiagnosis. Nearly half of men above the age of 50 years have small volume, latent prostate cancer in autopsy studies. This disease would not cause any morbidity or mortality during a man’s lifetime but is often inadvertently detected. The overdiagnosis rate of insignificant cancer is high when using a screening strategy where PSA is combined with transrectal ultrasound guided (TRUS) biopsy. The detection of this low-risk disease leads to men being subjected to the harm of a cancer diagnosis and the risk of side-effects from radical treatment, despite the ProtecT study showing treatment did not confer a survival benefit compared to observation over the 10-year follow-up [4].
Solving the problem of underdiagnosis
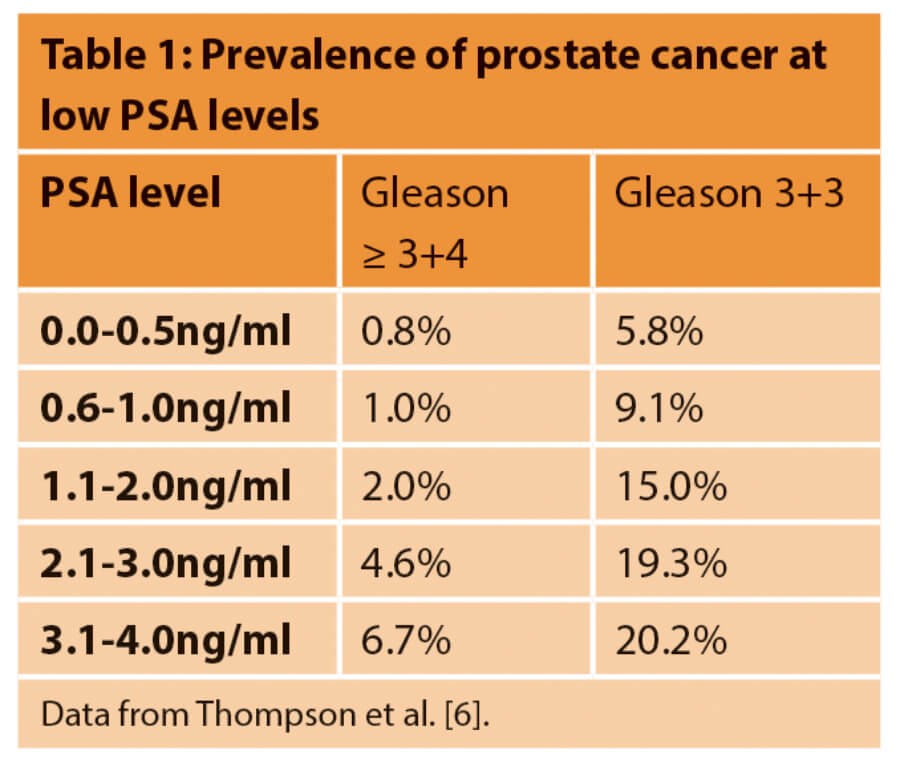
Even among men with PSA levels ≤4.0ng/ml, there is a risk of missing significant prostate cancer. In the Prostate Cancer Prevention Trial (PCPT), 2950 participants in the placebo group underwent a TRUS biopsy despite never having a PSA ≥4.0 or abnormal digital rectral examination (DRE) over the seven-year study period [6]. The results highlighted that significant prostate cancer is not rare among men with low PSA levels (Table 1). These results are based on random TRUS biopsy which has a high rate of missed diagnosis; with modern biopsy techniques using image fusion and intensive sampling from saturation or transperineal template technique it is likely that the detection of significant disease at low PSA levels would be even higher.
Moving beyond diagnosis to prognosis
Given the high prevalence of latent prostate cancer, we propose that screening should move towards a strategy of cultivating tests focused on prognosis rather than diagnosis. This requires the next generation of screening tests to be calibrated towards the detection of disease with a higher risk of progression to metastatic disease and death. The appropriate threshold for ‘significant disease’ remains under debate but there has been a steady trend towards less stringent definitions of significant disease including a proportion of Gleason pattern 4.
MRI has been shown to be capable of detecting significant disease with a high level of accuracy in men with a suspicion of prostate cancer. A recent Cochrane systematic review has reported a sensitivity of 91% across all studies which used template mapping biopsy [7]. This is in contrast to PSA in the PCPT trial where the sensitivity of PSA ≥3ng/ml was limited at 57.6%. There is no PSA threshold which can exclude significant prostate cancer and this fact has been highlighted in the recent European Association of Urology (EAU) prostate cancer guidelines which could not set a PSA specific threshold for triggering further investigations. This risk of missing lethal prostate cancer was highlighted in the CAP study which showed that 36% (68/188) of men who die from prostate cancer having attended the screening clinic had a one-off PSA of less than 3ng/ml.
Prostagram: a possibility or pipedream?
The prospect of a male equivalent of the breast mammogram or so-called ‘prostagram’ has been a long-standing goal in prostate cancer diagnostics. Image-based screening has been adopted for other common cancers including mammography for breast cancer and low dose CT for lung cancer screening. TRUS was touted as the original ‘male mammogram’ in the 1990s but did not prove to be effective as an independent test. The revolution of MRI has offered a new opportunity to re-explore this area. If the performance characteristics of a standard mpMRI can be replicated in the general population this would address some of the issues with PSA as a screening test.
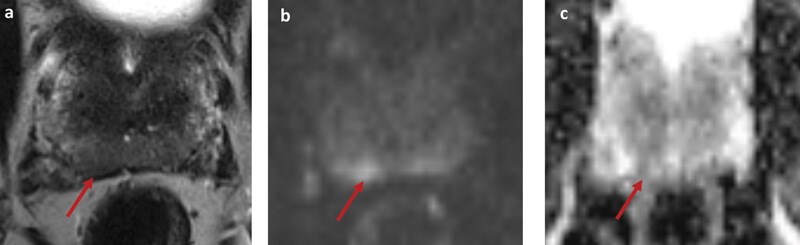
Figure 1: Example case; a ‘prostagram’ in a 57-year-old with PSA 1.02. He had no risk factors for prostate cancer and a benign DRE. A biparametric MRI (bpMRI) showed a basal right peripheral zone lesion with restricted diffusion on DWI (b) and corresponding hypointense signal on ADC (c). The lesion was score 4 out of 5 on PIRADS v2 and LIKERT scales. A targeted biopsy revealed Gleason 3+4 in all targeted cores with maximum cancer core length 7mm.
There are individual cases of MRI detecting significant disease in men with a low PSA and normal DRE (Figure 1). At present there have been limited studies evaluating MRI as a replacement for PSA. A small pilot study of MRI screening in 47 healthy volunteers has been completed by Nam et al. [8]. Although the sample size was small, the ROC curves suggested that MRI might have a higher diagnostic accuracy compared to PSA.
Screening tests are performed across a large population of asymptomatic individuals so need to be safe, simple and cost-effective. For a prostagram to be feasible for screening, it needs to be an accurate and simple MRI protocol which can be completed in less than 10 minutes without intravenous contrast. The conventional multi-parametric MRI protocol does not meet these criteria as it is expensive, takes 30-45 minutes and requires administration of gadolinium-based contrast. Although contrast-related adverse events are rare, these can become important when extrapolated across the population, particularly given current uncertainties around the impact of gadolinium deposition in the brain.
There are methods which would make MRI more suitable for screening by shortening MRI protocols, decreasing image acquisition times and removing the need for contrast. Techniques which improve MRI efficiency will lead to lower cost and increased capacity of MRI. In addition, the decreased time within the MRI scanner may improve acceptability of the test by minimising the time patients are lying in an enclosed narrow space.
There is an accumulation of evidence supporting the diagnostic performance of bi-parametric MRI (bpMRI) with no intravenous contrast. A recent meta-analysis by Kang et al. included 1705 patients from 10 studies comparing bpMRI with mpMRI [9]. The meta-analysis showed no statistically significant difference between the cancer detection rates of bpMRI and mpMRI.
Several studies have evaluated the use of abbreviated bpMRI protocols with acquisition times ranging from 10 to 15 minutes. The MULTI-IMPROD trial evaluated a 15-minute bpMRI protocol which included T2W imaging and diffusion weighted imaging performed on three different acquisitions [10]. It was a large, prospective multicentre cohort study where the target condition was Gleason score ≥3 + 4 determined by systematic biopsy +/- targeted biopsy. The sensitivity was 97% (CI 93%-99%) and negative predictive value 95% (CI 87%-98%).
Abbreviated bpMRI protocols may be further adapted to make MRI more suitable for mass population screening. Weiss et al. described an ultra-fast 3T protocol taking five minutes and consisting of axial T2-weighted with a simultaneous diffusion-weighted multislice EPI sequence [9]. This rapid protocol had a similar diagnostic accuracy to a standard mpMRI in 52 patients who completed this at one centre.
Looking to the future: the landscape of clinical trials
The proposition that MRI could be feasible as a screening test is based on outcomes extrapolated from a diagnostic population at risk of prostate cancer. It is unclear whether similar levels of diagnostic accuracy will be seen in the general population and there are various clinical trials which have commenced to address this question. The issue facing clinical trials in this area are the large sample sizes required due to the low event rate of prostate cancer in a healthy population.
The Imperial Prostate 1 (IP1) PROSTAGRAM (NCT03702439) and the ReIMAGINE (NCT04063566) studies are taking the initial steps by evaluating the acceptability of MRI and the prevalence of suspicious MRI lesions in the general population. A randomised controlled trial called ‘MRI Versus PSA in Prostate Cancer Screening’ is recruiting 1010 participants into PSA and MRI screening arms to compare detection of significant disease in Canada (NCT02799303).
Results are expected from these trials over the next few years but even if they show that MRI has higher detection rates for significant cancer, this does not necessarily translate into improved mortality outcomes. Given that we are focusing on prognosis rather than diagnosis, it is important that any new screening test is shown to be of benefit in a randomised controlled screening trial with mortality as the primary outcome.
Other trials which have started include the GÖTEBORG prostate cancer screening 2 trial which is randomising 40,000 men in Sweden to various arms (ISRCTN94604465). Rather than including MRI as a first-line test, it is being combined with PSA at a lower threshold of 1.8ng/ml. These trials will take many years to complete and the trial end date for the GÖTEBORG screening study is estimated as December 2040.
Meanwhile there are other novel biomarkers, such as the Stockholm-3 panel, Prostate Health Index, SelectMdx, Proteomedix and 4K score, which may improve the diagnostic accuracy of PSA. The Stockholm-3 panel is undergoing evaluation in a paired cohort study in primary care compared to PSA (NCT03381105). We should be cautious to avoid reaching conclusions about the efficacy of alternative screening tests for prostate cancer until the results of these trials are available. One of the lessons to be learnt from the PSA-era is that the early adoption of widespread population screening prior to evidence can cause unexpected harms.
Conclusion
An effective screening test for prostate cancer has the opportunity to have a significant impact on population health. Although MRI has characteristics which could make it an appealing tool for prostate cancer screening, there are challenges due to the high cost and limited availability of MRI scanners. The emergence of abbreviated bpMRI protocols are a step towards a technique which could be feasible for population screening. These new protocols are being investigated in clinical trials and the outcomes will provide an interesting insight into whether men could be getting a prostagram in the future.
References
1. UK NSC prostate cancer recommendation. 2016.
https://legacyscreening.phe.org.uk
2. Ten-minute scan may become universal screening tool for prostate cancer. The National Post 2019.
https://nationalpost.com/
health/10-minute-scan-may
-become-universal-screening
-tool-for-prostate-cancer
3. Lloyd T, Hounsome L, Mehay A, et al. Lifetime risk of being diagnosed with, or dying from, prostate cancer by major ethnic group in England 2008–2010. BMC Medicine 2015;13(1):171.
4. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. New England Journal of Medicine 2016;375(15):1415-24.
5. Hugosson J, Roobol MJ, Månsson M, et al. A 16-yr Follow-up of the European Randomized study of Screening for Prostate Cancer. European Urology 2019;76(1):43-51.
6. Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level≤ 4.0 ng per milliliter. New England Journal of Medicine 2004;350(22):2239‑46.
7. Drost FJ, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI‐targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database of Systematic Reviews 2019;4:CD012663.
8. Nam RK, Wallis CJ, Stojcic-Bendavid J, et al. A pilot study to evaluate the role of magnetic resonance imaging for prostate cancer screening in the general population. The Journal of Urology 2016;196(2):361‑6.
9. Kang Z, Min X, Weinreb J, et al. Abbreviated biparametric versus standard multiparametric MRI for diagnosis of prostate cancer: a systematic review and Meta-analysis. American Journal of Roentgenology 2019;212(2):357-65.
10. Weiss J, Martirosian P, Notohamiprodjo M, et al. Implementation of a 5-minute magnetic resonance imaging screening protocol for prostate cancer in men with elevated prostate-specific antigen before biopsy. Investigative Radiology 2018;53(3):186-90.
11. Jambor I, Verho J, Ettala O, et al. Validation of IMPROD biparametric MRI in men with clinically suspected prostate cancer: A prospective multi-institutional trial. PLoS Medicine 2019;16(6):e1002813.
All links accessed November 2019.
TAKE HOME MESSAGES
-
A population screening test for prostate cancer could have a significant impact on overall public health.
-
The dilemma for screening has been identifying an appropriate test which will detect the disease that causes harm without over-diagnosing insignificant disease.
-
MRI is a sensitive test for significant prostate cancer and has favourable characteristics and potential to address overdiagnosis and underdiagnosis caused by PSA screening.
-
Whilst there are legitimate concerns over its high cost and limited availability, recent technological advances have meant that a fast 5-10 minute MRI could be a feasible approach.
-
There are several new clinical trials which will provide interesting insights into the prospect of an abbreviated biparametric MRI protocol for screening men in the community.
Declaration of competing interests:
DEE receives research funding from the BMA Foundation for Medical Research, the Royal College of Surgeons, The Urology Foundation and Imperial Health Charity.
MW receives a travel grant and a loan of device from Zicom Biobot.
HA has received research funding from the Wellcome Trust, MRC, CRUK, Prostate Cancer UK, Sonacare Inc., Trod Medical, and Sophiris Biocorp. He is a paid medical consultant for Sophiris Biocorp, BTG/Galil and Sonacare Inc., and a paid proctor for HIFU cryotherapy and Rezum water therapy and is paid for training other surgeons in these procedures.






