The purpose of this article is to explain and illustrate common renal vascular variants that can be depicted with imaging. Renal vessels commonly present a wide range of variations [1]; before major renal or vascular surgery is undertaken, accurate portrayal of the renal vasculature is vital. Examples of the surgeries and procedures requiring vascular mapping include: donor renal nephrectomy, partial nephrectomy, vascular treatment for renal artery stenosis, inferior vena cava (IVC) filter placement, and open surgical or endovascular treatment for abdominal aortic aneurysm [2,3].
Imaging modality
Traditionally, conventional catheter angiography was used to assess renal vascular anatomy. However, this invasive procedure has now been largely replaced with CT angiography (CTA) as the principal imaging investigation for renal vasculature assessment [3]. Volume rendered CT allows clear and accurate delineation of the origin and course of renal vasculature without need for an invasive procedure. The sensitivity of this study approaches 100% with arterial vessels identified to at least the segmental level [4]. CTA additionally surpasses conventional angiography with higher quality venous anatomy depiction [3]. Overall surgical and CT findings correlate in greater than 95% of patients [4].
MR angiography is an alternative to CTA, and avoids ionising radiation, however its spatial resolution is inferior to CTA, the imaging is more costly, and less easily available [3]. In this pictorial review therefore the renal vascular variants will be displayed with the gold standard CTA as currently used in practice.
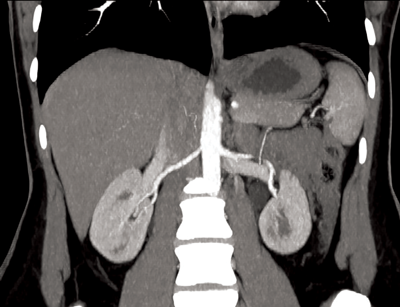
Figure 1: Coronal CTA demonstrating single renal artery supplying
each kidney and single vein draining each kidney.
Normal renal vascular anatomy (Figure 1)
Approximately 70% of the population have single bilateral renal arteries, originating from the abdominal aorta, at the superior margin of the second lumbar vertebral body, slightly below the superior mesenteric artery (SMA) origin [3]. The right renal artery orifice is located on the anterolateral wall of the aorta. The left renal artery originates slightly more inferolaterally than the right [2]. The typical dimensions of the renal arteries are 4-6cm in length and 5-6mm in diameter.
The renal arteries have slightly different trajectories, although both pass posteriorly towards the renal hilum. The right renal artery courses downward, and passes behind the IVC, while the left renal artery courses more horizontally and slightly upwards, posterior to the left renal vein. In the majority of cases the inferior adrenal arteries arise from the main renal arteries, and from the proximal aspect in two thirds of patients [2]. The main renal artery divides into segmental arteries approaching the renal hilum. The first division is typically the posterior branch, which passes posterior to the renal pelvis, to supply the posterior portion of the kidney. The remaining main renal artery then continues before dividing into four anterior branches at the medial aspect of the renal hilum: the apical, upper, middle, and lower anterior segmental arteries [4].There is no collateral arterial circulation between the renal segments; each of the segments is supplied by its own segmental artery [1]. Therefore segmental renal infarction will occur in the case of accidental segmental artery ligation.
Renal veins course anterior to the renal arteries. The segmental veins converge to form the main renal vein at the renal hilum [2]. The left renal vein usually receives the left adrenal, gonadal and lumbar veins before passing between the aorta anteriorly and superior mesenteric artery posteriorly, to enter the IVC. The shorter right renal vein, has no tributaries and enters the IVC at the level of first lumbar vertebra [3]. The right renal vein averages 2-4cm in length. The left renal vein averages 6-10cm in length [4]. In contrast to the segmental arteries there is free anastomosis of the venous system in the kidneys. This allows for ligation of the venous branches if a vessel is damaged during surgery, permitting an alternative venous drainage [1].
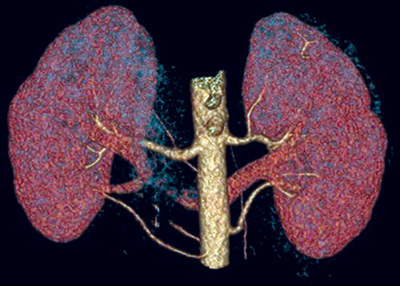
Figure 2: 3D reconstruction of a CTA showing two left renal arteries and three right renal arteries.
Renal artery variants
Accessory renal arteries (Figure 2)
Unilateral accessory renal arteries can be seen in approximately 30% of patients, with bilateral accessory arteries in approximately 10%, making it the most common renal arterial variation. The common origin for accessory arteries is the aorta or common iliacs between the levels of T11-L4. More rarely the origin may be the lower thoracic aorta, lumbar or mesenteric arteries [4]. Typically the accessory artery passes into the renal hilum and perfuses either the upper or lower renal poles. These hilar accessory arteries are similar in diameter to the main renal artery. However a further subtype exists where the accessory renal artery may enter directly into the renal parenchyma directly from the cortex, commonly at the renal pole; these accessory renal arteries are given the nomenclature polar arteries.
They have the same variety of origin as the hilar accessory renal arteries, but also differ in that the polar arteries have a smaller calibre [2]. The most common accessory artery is a polar artery originating from the aorta, near the main renal artery, and supplying the inferior renal pole. It is important to note that some authors give the nomenclature polar arteries if the vessel enters at the renal pole, irrespective of origin, i.e. the ‘polar artery’ may be an aberrant branch of the main renal artery, and not an accessory artery at all. Regardless of this nomenclature overlap it is important to identify polar arteries as they provide a segmental arterial supply, and if damaged can cause arterial bleeding and, or indeed also renal infarction [1].
Prehilar or extrahilar branching
Prehilar branching is the branching of the main renal artery into segmental arteries proximal to the renal hilum. If this occurs within 2cm of the renal artery origin it is termed early branching. This is an essential arterial variant to identify, as surgeons require at least a 2cm length of renal artery before hilar branching to guarantee satisfactory control and anastomosis. Early branching of the left renal arteries has been observed in 21% of cases, and in 15% of cases for right renal arteries [1].
Renal vein variants
Supernumerary renal veins
These are seen in between 15-30% of patients, but are rare on the left side [2]. On the right side there may be as many as four main right renal veins entering the IVC. In 4% of patients the right renal vein branches to let the right gonadal artery pass through [1]. In 6% of cases the right gonadal veins directly join the right renal vein, rather than the IVC. In 3% of cases this is also true for retroperitoneal veins. It is important to note however, that there are multiple venous anastomosis in the kidneys, and therefore the non-dominant renal vasculature can be sacrificed if surgically necessary [3].
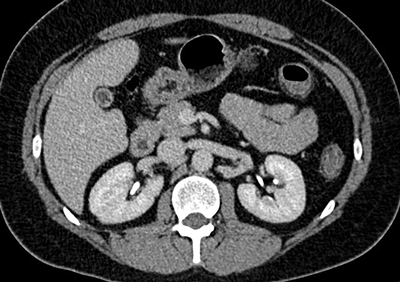
Figure 3: Two left renal veins, one seen anterior to the aorta
and the second seen coursing posterior to the aorta.
Circumaortic renal vein (Figure 3)
There are two varieties of circumaortic renal vein, which overall have a prevalence up to 8.7% [5]. Most commonly the left renal vein bifurcates into two limbs just prior to reaching the abdominal aorta and thus enclosing it [2]. In the remaining 25% the left renal vein duplicates at the renal hilum. The retroaortic limb is positioned more caudally to the preaortic limb, and is often the smaller limb [4]. Usually, the gonadal vein joins the dorsal left branch of the left renal vein, whereas the adrenal vein joins the ventral branch [1].
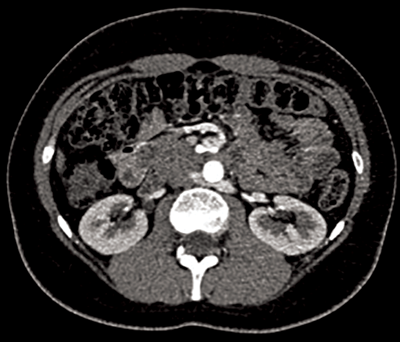
Figure 4: Retroaortic left vein seen posterior to the aorta.
Retroaortic renal vein (Figure 4)
This is less common than the circumaortic renal vein, with a prevalence of 2.1%. The left renal vein passes posterior to the aorta and drains into the lower lumbar aspect of the IVC, or more rarely the common iliac vein [1,5].
IVC variants
The inferior vena cava can display a number of anatomical variants; those specifically affecting the renal vasculature are described below.
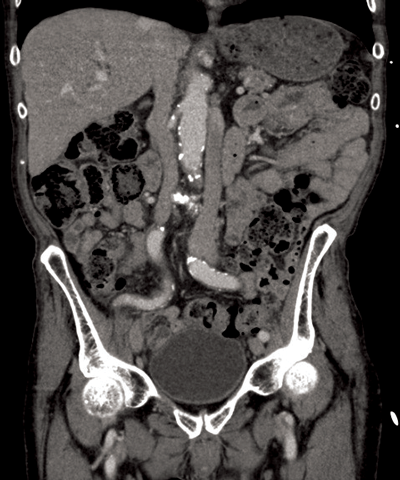
Figure 5: Two IVCs are seen, one either side of the aorta.
Double IVC (Figure 5)
A left and right IVC is present inferior to the renal vein. The prevalence is 0.2-3% of patients. Typically the left IVC ends at the left renal vein, which then continues its typical course, crossing anterior to the aorta to join the right IVC. There may be significant size asymmetry between the two IVCs. Double IVC should be suspected in cases of recurrent pulmonary embolism following placement of an IVC filter. Note in rare cases this variant can be combined with a retroaortic left renal vein [5].
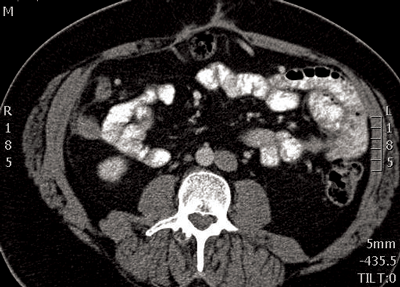
Figure 6: IVC seen adjacent to the left side of the aorta.
Left IVC (Figure 6)
As with the double IVC the left IVC joins the left renal vein. The left renal vein crosses anterior to the aorta to unite with the right renal vein, forming a right-sided suprarenal IVC. The prevalence is 0.2-0.5% of patients. Prior knowledge of this vascular variant is important, as transjugular access to the infrarenal IVC for placement of an IVC filter may be difficult [5].
Duplication of IVC with retroaortic right renal vein and hemiazygos continuation of IVC
In this variant there are duplicate IVCs infrarenally. The right IVC ends at the right renal vein, which then crosses posterior to the aorta. The retroaortic right renal vein then joins the left IVC. The left IVC then passes posteriorly to the diaphragmatic crus to enter the thorax as the hemiazygos vein. In the thorax, the hemiazygos vein crosses posterior to the aorta at approximately T8 or T9 to join the azygos vein. This double variant must be identified preoperatively as perioperative death following inadvertent ligation of hemiazygos-to-azygos continuation of a left IVC during thoracic surgery has been reported [5].
Absent infrarenal or entire IVC
The external and internal iliac veins join to form enlarged ascending lumbar veins. Venous return from the lower extremities passes into the azygos and hemiazygos systems via anterior paravertebral collateral veins. In some cases a suprarenal IVC is formed by the merging of the left and right renal veins. Patients with absent IVC may present with symptoms of lower-extremity venous insufficiency or idiopathic deep venous thrombosis [5].
Conclusion
Accurate depiction of the renal vasculature is vital prior to numerous surgical and interventional radiology procedures to avoid inadvertent damage to the kidneys, commonly through renal infarction. CT angiography is currently the gold standard imaging technique for preoperative planning, and this pictorial review has displayed some of the anatomical variants that may be demonstrated.
References
1. Arévalo Pérez J, Gragera Torres F, Marín Toribio A, et al. Angio CT assessment of anatomical variants in renal vasculature: its importance in the living donor. Insights into Imaging 2013;4:199-211.
2. Hazırolan T, Öz M, Türkbey B, et al. CT angiography of the renal arteries and veins: normal anatomy and variants. Diagn Interv Radiol 2011;17:67-73.
3. Türkvatan A, Ozdemir M, Cumhur T, Olçer T. Multidetector CT angiography of renal vasculature: normal anatomy and variants. European Radiology 2009;19:236-44.
4. Urban BA, Ratner LE, Fishman EK. Three-dimensional volume-rendered CT angiography of the renal arteries and veins: normal anatomy, variants, and clinical applications. Radiographics 2001;21:373-86.
5. Bass JE, Redwine MD, Kramer LA, et al. Spectrum of congenital anomalies of the inferior vena cava: cross-sectional imaging findings. RadioGraphics 2000;20:639-52.