Castrate resistant prostate cancer (CRPC) is defined by disease progression despite androgen-deprivation therapy lowering testosterone to castrate levels. It may present as a rise in serum levels of prostate specific antigen (PSA), progression of pre-existing disease, or the appearance of new metastases. The management of castrate resistant prostate cancer has evolved over the last decade.
In 2004, docetaxel became the first treatment to show a survival benefit and emerged as standard of care in CRPC [1]. However new therapeutic options have expanded rapidly since 2011 including second-line chemotherapy, second generation hormonal targeted therapy and bone seeking radionuclides. In addition, bisphosphonates and denosumab have decreased skeletal related adverse events, whilst improving quality of life.
Over the last five years, treating men with CRPC has become more challenging, as there are more available treatment options including clinical trials examining sequencing or combination of treatment. This review will summarise the approved available therapeutic options in patients with metastatic castrate resistant prostate cancer.
Androgen deprivation therapy (ADT)
ADT remains the standard systemic therapy in locally advanced and metastatic prostate cancer. This is for both castration sensitive and resistant disease as the androgen receptor remains functionally active in both disease states. In 1941 Huggins and Hodges first demonstrated regression of prostate carcinoma through endocrine control [2]. ADT decreases circulating testosterone to castrate levels resulting in lower cancer cell proliferation and subsequently induction of apoptosis, but does not typically eradicate the cancer.
There are two forms of medical ADT, luteinising hormone-releasing hormone (LHRH) agonists and antagonists. LHRH analogues stimulate LH receptors in the pituitary gland leading to LH receptor down regulation, resulting in castration in two to four weeks. Testosterone may rise in first two to three weeks, with a risk of tumour flare up, therefore co-treatment with an antiandrogen is started two weeks prior to the initiation of LHRH agonists. Ninety percent of men respond with a median duration of 12-18 months [5]. Equally LHRH antagonists bind to LHRH receptors in the pituitary gland, resulting in rapid and profound testosterone suppression. They are used predominately in patients at risk of spinal cord compression or with a high burden of symptomatic disease.
Chemotherapy
Docetaxel
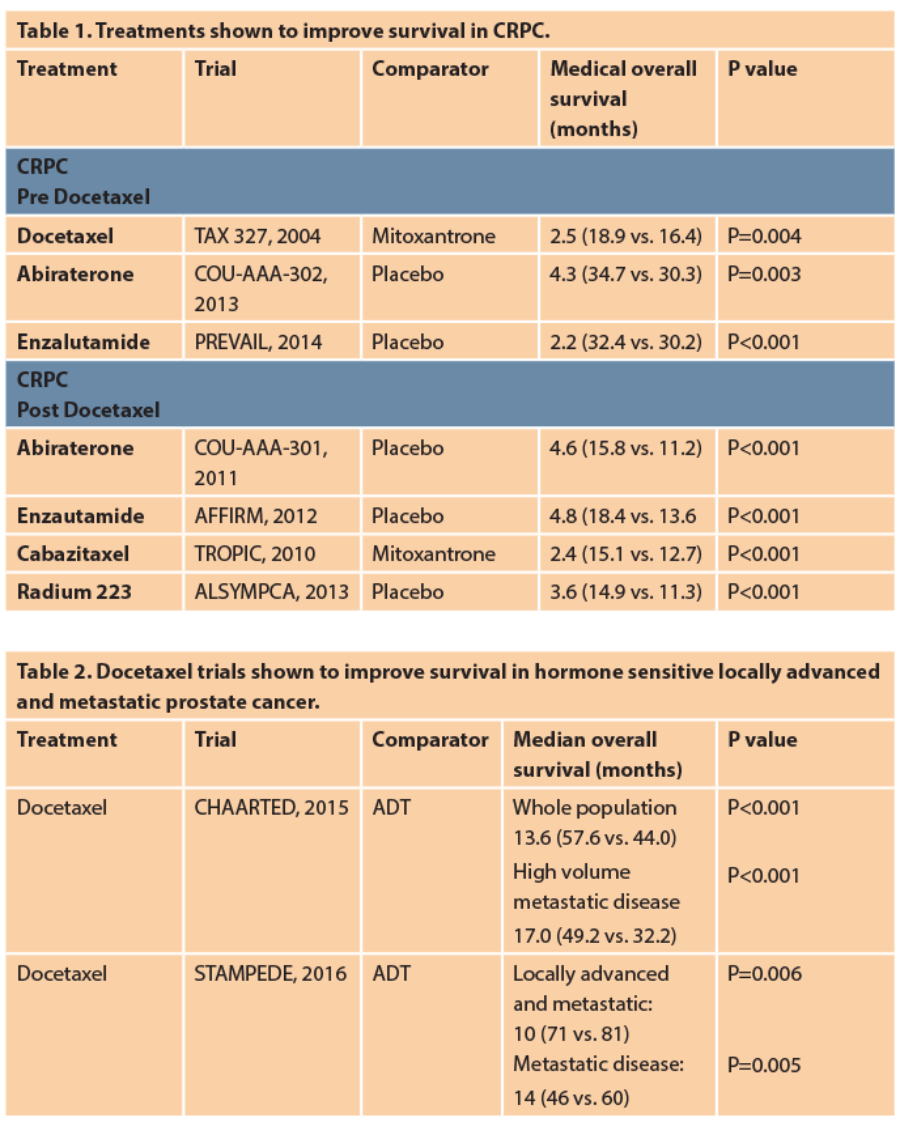
Docetaxel is a microtubule inhibitor, it is given intravenously every three weeks, up to a maximum of 10 cycles, lasting 30 weeks in metastatic CRPC. It was approved and licensed as first-line therapy for metastatic CRPC in 2004 following the results of the TAX-327 trial [3]. The TAX 327 trial compared docetaxel with mitoxantrone demonstrating a 2.5-month median survival benefit (18.9 vs. 16.4 months). Docetaxel was associated with higher incidence of grade three to four toxicities, however it also showed significant reduction in pain by 35%.
Docetaxel is now being used earlier in patients with hormone sensitive metastatic prostate cancer, based on the results of the CHAARTED and STAMPEDE trials. In 2014 the CHAARTED study reported a survival benefit of 14 months (58 vs. 44 months) when docetaxel was added to ADT in men with metastatic hormone sensitive prostate cancer [4]. In 2015 the STAMPEDE trial showed similar results with an associated survival benefit of 10 months for the addition of docetaxel to ADT in men with both locally advanced and metastatic hormone sensitive prostate cancer (81 vs. 71 months). In the subgroup analysis in metastatic disease the survival benefit was significantly longer at 15 months (45 vs. 60 months) [5]. Given this compelling data, treatment has now changed to incorporate docetaxel.
The common side-effects of docetaxel are peripheral sensory neuropathy, neutropenia, febrile neutropenia, diarrhoea, nausea and fatigue; treatment is generally better tolerated on hormone sensitive setting as patients are fitter. Increased incidence of neutropenia noted in 12% and febrile neutropenia in 15% .
Second generation androgen receptor targeted therapies
Abiraterone
Abiraterone inhibits the androgen synthesis pathway though blockade of 17-a-hydroxylase and lowers the testosterone levels more than ADT alone. It is an oral drug and is given with low dose oral prednisolone to minimise the side-effects of mineralocorticoid excess. The COU-AA-302 trial, which compared abiraterone plus prednisolone against placebo plus prednisolone in men with metastatic CRPC without previous chemotherapy, showed significant longer overall survival benefit of 4.3 months (34.7 vs. 30.3 months) [6]. The COU-AA-301 trial, which compared abiraterone plus prednisolone versus placebo with prednisolone in men with metastatic CRPC with previous chemotherapy, showed overall survival benefit of 4.6 months (15.8 vs. 11.2 months). In the sub-analysis, pain control was significantly better in the abiraterone group (45% vs. 28.8%), with faster time to palliation of pain (5.6 vs. 13.7 months) [7].
Abiraterone is licensed and NICE approved in pre and post docetaxel setting. It is well tolerated with rare side-effects of fatigue, fluid retention, hypertension, cardiac disorders, hypokalaemia and liver dysfunction.
Enzalutamide
Enzalutamide is an oral androgen receptor inhibitor, which inhibits nuclear translocation and impairs the androgen receptor binding to DNA thus preventing gene expression and growth, survival and proliferation of prostate cancer cells. Trials conducted for this second generation anti-androgen agent, all showing superiority to either placebo or bicalutamide, pre and post chemotherapy. The AFFIRM trial compared enzalutamide versus placebo in men with metastatic CRPC with previous chemotherapy. The overall survival benefit was significantly longer at 4.8 months (18.4 vs. 13.6 months). There was also significant improvement in pain progression and quality of life in the enzalutamide arm [8]. The PREVAIL trial compared enzalutamide with placebo in men without previous chemotherapy. The overall survival benefit was 2.2 months (32.4 vs. 30.2 months).
There was also an impressive improvement in progression free survival (PFS) of 81% [9]. Recent data published from the TERRAIN study showed an improvement in PFS of 9.9 months (15.7 v 5.8 months) with enzalutamide versus bicalutamide in men with CRPC who progressed on ADT [10]. The STRIVE study showed decreased risk of prostate progression or death by 76% for enzalutamide versus bicalutamide in men with metastatic or non-metastatic prostate cancer and mean PFS 19.4% vs. 5.7% [11]. There are other ongoing studies looking into the role of enzalutamide in non-metastatic CRPC.
Enzalutamide is licensed and NICE approved in the pre and post docetaxel setting. It is well tolerated with most common side-effects of fatigue, hot flushes, diarrhoea, musculoskeletal pain, headache and hypertension. In the AFFIRM study a low incidence of seizures was reported (0.6%).
Other systemic anti-cancer therapies
Cabazitaxel
Cabazitaxel is a second generation taxane, licensed as second-line chemotherapy after docetaxel. The TROPIC trial compared cabazitaxel against mitoxantrone plus prednisolone in men with metastatic CRPC and showed an overall survival benefit of 2.4 months in the cabazitaxel arm (15.1 vs. 12.7 months) [12]. The side-effects were similar to docetaxel but with a higher incidence of neutropenia noted, grade 3-4 in 82% and febrile neutropenia in 8%.
Radium 223
Radium 223 is an alpha-emitting, bone seeking, calcium mimetic that selectively targets and binds to increased areas of bone turnover in bone metastases. The ALSYMPCA trial compared radium 223 against placebo in patients with CRPC and bone metastases. Median overall survival was 3.6 months longer (14.9 vs. 11.3 months) [13]. A significant improvement in median time to first symptomatic skeletal event was also seen in the radium 223 arm (15.6 vs. 9.8 months) and an overall improvement in quality of life. The most common side-effects reported are: diarrhoea, nausea, peripheral oedema and bone marrow suppression.
Radium 223 is licensed and approved by NICE for patients who have previously been treated with docetaxel and available via the Cancer Drugs Fund for patients who are not fit for docetaxel or who have declined chemotherapy. It is licensed only in patients with bone (two or more bones) and non-visceral metastatic disease. Radium 223 is given intravenously once every four weeks for six months in commissioned centres in collaboration with the treating oncologist and nuclear medicine specialists.
Role of biphosphonates and bone targeted therapy
Ninety percent of patients with metastatic CRPC have bone metastases and significant risk of skeletal events. The role of zolendronic acid and denosumab to prevent adverse skeletal events and improve pain and quality of life has proven to be beneficial. Zometa is a bisphosphonate and denosumab, a human monoclonal antibody directed against RANK ligand to inhibit osteoclast mediated bone destruction. A study comparing zometa versus denosumab showed a slightly longer time to skeletal related events with denosumab (20.7 vs. 17.1 months) [14].
Denosumab is administrated as a subcutaneous injection 120mg every four weeks, with a higher risk of hypocalcaemia when compared with zometa, which is administrated intravenously every three to four weeks. The latter has a risk of renal impairment and both agents a similar risk of osteonecrosis of the jaw.
Future
Mechanisms of resistance
Recent evidence has suggested drug resistance in patients who do not respond to abiraterone or enzalutamide, may be associated with the androgen receptor variant: AR-V7. A prospective study showed that 39% of patients treated with enzalutamide and 19% with abiraterone had detectable AR-V7 in their circulating tumour cells [15]. Other studies have showed that patients not responding to abiraterone may have androgen receptor gene aberrations (duplication of a specific nucleotide sequence), however patients with AR-V7 variant may respond better to docetaxel but further studies need to be conducted to determine if treatment decisions based on the molecular profile will improve outcomes. Therefore, when there is no satisfactory PSA response to selected treatment, we need to consider the underlying possibility of resistance and stop treatment earlier.
DNA repair abnormalities
The recent phase two TOPARP looked at the poly-adenosine diphosphate ribose polymerase (PARP) inhibitor, olaparib, in metastatic CRPC. It showed increased activity in men with defects in DNA repair genes who have not responded to previous therapies [16]. The results are exciting as they support personalised treatment and the role of PARPi is being investigated in phase three studies in CRPC.
Immunotherapy / other targeted therapies
Immunotherapy studies have so far failed to show promising survival results. Other targeted therapies such as cabozantinib, a tyrokinase inhibitor, have shown promising results in men with CRPC, previously treated with docetaxel in phase two study but this result was not mirrored in a phase three trial.
Combination / sequential treatment
Other studies are examining the combination of different treatments, such as dual AR blockade with enzalutamide and abiraterone. In addition other trials are looking at different sequences of drugs, such as combining enzalutamide with docetaxel [17] early in the treatment pathway.
Conclusion
Important advances have been made in the treatment of CRPC since docetaxel approval in 2004. All these new agents have shown a median survival benefit of two to five months. A systematic approach needs to be followed to personalise treatment based on burden of the disease, presence of visceral or non-visceral bone metastases, degree and severity of symptoms, performance status and medical comorbidities, previous use of docetaxel chemotherapy and side-effect profile. Molecular profiling will aid the ability to tailor therapy and improve further survival and quality of life. Treatment decision is challenging, therefore patients should be managed in specialist multidisciplinary teams and offered entry into clinical trials that help answer these questions.
References
1. Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20.
2. Huggins C, Hodges CV. Studies on prostatic cancer II: the effects of castration on advanced carcinoma of the prostate gland. Arch Surg 1941;43:209-23.
3. Tannock IF, de Wit R, Berry WR, et al. TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12.
4. Sweeney C, Chen Y, Carducci M, et al. Chemohormonal therapy in metastatic hormone sensitive prostate cancer. N Engl J Med 2015;373:737-46.
5. James ND, Sydes MR, Clarke NW, et al. STAMPEDE investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2016;387:1163-77.
6. Ryan CJ, Smith MR, Fizazi K, et al. COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152-60.
7. Fizazi K, Scher HI, Molina A, et al. COU-AA-301 Investigators. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double- blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983-92.
8. Scher HI, Fizazi K, Saad F, et al. AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97.
9. Beer TM, Armstrong AJ, Rathkopf DE, et al. PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424-33.
10. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol 2016;17:153-63.
11. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide
versus bicalutamide in castration-resistant prostate cancer: The STRIVE trial. J Clin Oncol 2016;34:2098-106.
12. de Bono JS, Oudard S, Ozguroglu M, et al. TROPIC Investigators. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration- resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147-54.
13. Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel
use: a prespecified subgroup analysis from the randomised, double- blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014;15:1397-406.
14. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration- resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813-22.
15. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028-38. 16. Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 2015;373:1697-708.
17. Lorente D, Mateo J, Perez-Lopez R, et al. Sequencing of agents in castration-resistant prostate cancer. Lancet Oncol 2015;16:e279-92.
TAKE HOME MESSAGE
-
Androgen deprivation therapy remains the mainstay of systemic treatment for metastatic prostate cancer.
-
New therapeutic options have expanded rapidly including second-line chemotherapy, second generation hormonal targeted therapy and bone seeking radionuclides.
-
Denosumab and biphosphonates have decreased skeletal adverse events and improved quality of life.
-
PARP inhibitors have shown initial promise in patients with defects in DNA repair genes.
-
Sequential or combination therapy studies aiming to overcome resistance are currently ongoing.
Declaration of competing interests: None declared.