Prostate cancer is the commonest cancer and the second most frequent cause of cancer death in Western men [1]. The recent STAMPEDE data suggests a median survival of just 42.1 months in the control arm of metastatic men [2]. Current standard-of-care consists of androgen deprivation therapy (ADT) +/- chemotherapy based on the STAMPEDE and CHAARTED studies [2,3].
However, there are emerging data that radical therapy directed at the prostate impacts survival, especially in those with limited metastatic burden or oligometastases, defined as one to three skeletal lesions without any visceral metastases [4,5]. Furthermore, many men suffer symptomatic disease progression and eventually require palliative surgical intervention, which is less frequent in those treated with initial radical prostatectomy compared to systemic therapy alone [6,7]. Hence, we ultimately aim to examine whether radical prostatectomy can impact survival and quality-of-life in men with oligometastatic prostate cancer.
There are convincing data to support the concept of radical therapy in many metastatic cancers, as detailed in our recent review [4]. For instance, a meta-analysis of 6885 advanced ovarian carcinoma patients and a recent Cochrane review have concluded that there is a clear survival benefit with debulking of the primary tumour. Within urology, The European Organisation for Research and Treatment of Cancer (EORTC) and SWOG studies have demonstrated that nephrectomy before systemic therapy improves one-year survival by 13-26% compared to systemic therapy alone, and to perform a nephrectomy in patients with metastatic renal cell carcinoma is standard-of-care. Observational data also support the use of radical therapy for glioblastoma, peritoneal carcinomatosis from gastrointestinal cancer, and metastatic colon cancer.
As well as data from other tumour types, there is a strong biological rationale for considering a radical approach to metastatic prostate cancer, also discussed in our recent review [4]. The ‘seed and soil’ hypothesis postulates that a receptive microenvironment (the ‘soil’) allows disseminating malignant cells (the ‘seed’) to engraft into and form metastases with soil development thought to be driven by factors secreted by the primary tumour. There is evidence that the primary tumour can seed to distant sites and cancer cells at those end-sites can further seed the primary lesion, leading to a vicious circle of metastasis; this ‘self-seeding’ phenomenon is dependent on the presence of an intact primary focus. Also, we know that disseminated tumour cells in men with clinically localised prostate cancer before prostatectomy confer a five-fold increased risk of future metastases but the same burden of these cells detected after surgery do not increase that risk [8]. Hence, it appears that the primary lesion has a role in driving metastatic progression.
Despite the above biological data and the trials and observational evidence from other cancers, there are as yet no published prospective studies that directly evaluate the role of cytoreductive surgery in advanced prostate cancer. Recent observational cohort studies from the US Surveillance, Epidemiology, and End Results (SEER) database and the Munich Cancer Registry found that men with metastatic prostate cancer treated with radical therapy had higher five-year survival than those treated with systemic therapy alone [9,10]. We also recently showed that at least 1206 men in Sweden have been treated with initial radical therapy (surgery or radiotherapy) for likely metastatic or micro-metastatic prostate cancer from 1996-2010, and on further interrogation of 18,352 cases found that men who underwent initial ADT without radical therapy were approximately three-times more likely to die of prostate cancer than those that had radical therapy (Eur Urol, in press).
So if we accept that there are enough biological and epidemiological data to warrant studying the hypothesis that radical therapy impacts survival in men with newly diagnosed metastatic prostate cancer, the next question is what radical therapy modality should we interrogate. There are no prospective data to inform this choice currently, so we have to extrapolate from the metachronous (recurrent) metastatic disease setting. A subgroup analysis of SWOG 8894 on 1286 men with metastatic prostate cancer showed a reduced risk of death in those who had previously undergone radical prostatectomy compared to those who had not [11].
Another study of 161 men who all received ADT for failure post-radical therapy showed that time to subsequent failure after ADT was longer in the surgical cohort than the radiotherapy one [12]. A report of 916 men with metastatic prostate cancer that originally received either radical prostatectomy or radiation for clinically localised disease also showed a substantial reduction in prostate cancer mortality rates for the surgically-treated group [13]. It may therefore be that surgery might be a good choice for the radical treatment modality to be used in prospective studies.
Although there is one ongoing feasibility trial in metastatic prostate cancer using radical prostatectomy (NCT01751438), this US-based study offers men a choice of surgery or radiotherapy and is thus not truly randomised. The only two ongoing randomised trials worldwide (STAMPEDE-NCT00268476; HORRAD-NTR271) are using radiation as the radical modality [14]. This is partly due to concerns regarding the safety of radical prostatectomy in this setting and partly due to a failure of the urological community to adequately engage with this question. In order to address the first concern, we compiled a cohort of 106 men from the United States, Germany, Italy, and Sweden who underwent radical prostatectomy for known newly-diagnosed metastatic disease and found similar rates of re-operations, re-admissions, transfusions, and 21 specific complications as in our previous meta-analysis on 286,876 men after radical prostatectomy for ‘conventional’ indications [15,16].
So if we accept that surgery to the primary tumour should be investigated in metastatic prostate cancer, the next question is which men should be included. Do we really think that the benefit for surgery might be seen in men with a super-scan of metastatic burden, or, perhaps more likely, will any benefit be confined to those with limited systemic disease? The recent landmark CHAARTED study demonstrated that men presenting with oligometastatic prostate cancer (≤ three skeletal lesions) have improved overall survival (and are probably less chemo-responsive) than those with polymetastatic disease (> three skeletal deposits) [3], and thus oligometastatic disease might represent a transitory disease phenotype.
Furthermore, all the above observational data in support of radical prostatectomy for metastatic disease are heavily confounded by selection bias with those undergoing radical treatment likely having fewer skeletal metastases than those undergoing ADT alone; a subgroup analysis of the SEER data supports this contention [17]. Hence, if there is a true survival benefit for radical therapy it is likely to be confined to cases with a lower metastatic burden.
Thus, if we are to interrogate the question of radical therapy for metastatic cancer, then surely it would make sense to limit the cohort to oligometastatic men, at least in initial studies. However, STAMPEDE (UK), HORRAD (Netherlands), and NCT01751438 (USA) are not only using radiotherapy as their local therapy modality but are also lumping all metastatic men together, with no distinction between oligo and polymetastatic disease. While other study proposals for oligometastatic prostate cancer specifically are in development by other investigators, these are focused on metachronous disease (in which men have recurrent oligometastatic cancer rather than at presentation), and thus they all involve treating the metastatic sites themselves (metastasis-directed therapy; MDT) [18,19].
The impact of treating the oligometastatic sites with stereotactic body radiotherapy (SBRT) in recurrent prostate cancer evaluates a different research question and the UK CORE trial will examine this for a number of tumour types including prostate.
Therefore, there is an urgent unmet need for a randomised controlled trial examining the impact of surgery in men with newly-diagnosed oligometastatic prostate cancer. ‘Testing Radical prostatectomy in men with prostate cancer and oligoMetastases to the bone: a randomised controlled feasibility study’ (TRoMbone) aims to randomise 50 men to standard-of-care (ADT with / without docetaxel) versus standard-of-care plus radical prostatectomy with extended pelvic lymphadenectomy. All modalities of surgery (open, laparoscopic, and robotic) are allowed and a quality assurance programme is built in, mandating only one surgeon per centre perform these cases, evidence of >100 cases performed prior to the study, and a median lymph node yield >10 nodes using a standardised templated dissection (common iliacs, external iliacs, internal iliacs, obturators, fossae of Marcille).
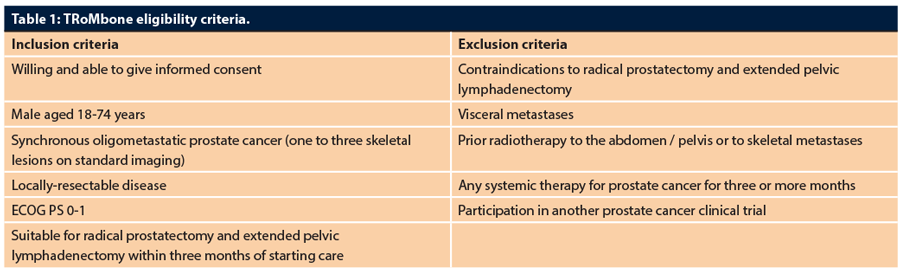
Men who are stage M1b with one to three skeletal metastases, age <75, ECOG PS 0-1, and with locally-resectable disease will be eligible for TRoMbone. The choice of staging modality will be as per standard clinical care, with more sophisticated imaging such as PSMA- or choline- PET allowed but not needed for eligibility. A grant application for an imaging sub-study on TRoMbone patients comparing imaging modalities is being developed currently, as the global community is divided as to the definition of oligometastatic disease and what modality is used for that definition. Further, access to treatment-light radical prostatectomy tissue (we estimate the average time from start of systemic therapy to surgery in the study will be less than six weeks, and is mandated to be less than three months) from men with oligometastatic prostate cancer is highly valuable for molecular research. We thus plan to bio-bank the samples and are setting up a translational committee that will review proposals for translational work using these samples from interested investigators.
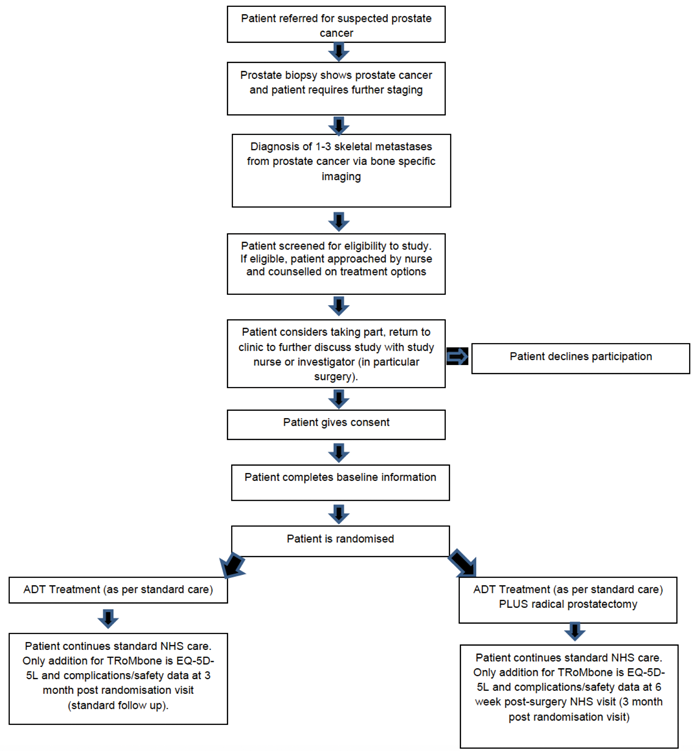
Figure 1: The patient pathway in the TRoMbone study.
Three centres will run TRoMbone: Oxford (Principal Investigator (PI) Freddie Hamdy), the Royal Surrey County Hospital (PI Christopher Eden), and University College London Hospital (PI John Kelly, Chief Investigator (CI) P Sooriakumaran). The study will be managed by the Surgical Intervention Trials Unit (SITU) at the University of Oxford (Operational Lead Surjeet Singh). The patient pathway and eligibility criteria are shown in Figure 1 and Table 1.
TRoMbone has two main challenges: (1) the ability to identify eligible patients, and (2) the ability to randomise these men to the study treatments. The current American Joint Committee on Cancer (AJCC) tumour-node-metastasis (TNM) staging system of prostate cancer groups all skeletal-metastatic patients together as M1b and there are no official statistics as to numbers presenting with newly diagnosed oligometastatic prostate cancer. Therefore, we conducted a prospective audit of 12 geographically diverse UK cancer centres over a three-month period and found that roughly 20% of newly diagnosed skeletal-metastatic patients present with oligometastases. None of the current randomised trials are recording the number of skeletal metastases and thus, while our proposal represents a novel opportunity to evaluate response specifically in the oligometastatic population, it will require a change in imaging reporting practice to identify these patients. MDTs across the land routinely comment on the presence of metastatic disease without defining extent of metastatic burden, and hence for TRoMbone to succeed, a shift in how we describe metastatic prostate cancer in the UK is required. We need to start separating metastatic disease into oligo- and poly- subgroups.
The original power calculation based on a survival primary endpoint required over 400 subjects, and thus we developed an international study (CI Graefen, Wiklund, Sooriakumaran) of which TRoMbone was planned as the UK arm (CI Sooriakumaran). The international study has opened in Germany, Sweden, and Austria and, despite being able to identify large numbers of eligible patients, is recruiting slowly due to a lack of equipoise and acceptance of uncertainty as to relative benefits of the treatment options (NCT02454543). This ability to randomise represents the second major challenge to TRoMbone’s success and is by no means unique to this question. So many high-profile prostate cancer trials have failed to recruit. And a surgical study on colorectal cancer and pulmonary metastases (PulMiCC) that was on the National Cancer Research Institute (NCRI) Portfolio and had the wide support of the surgical community has also recently failed to recruit and been closed down [20].
So, before commencing a full trial examining survival, we need to assess the feasibility of our proposed study in the UK. And we need to learn lessons from the surgical RCTs that have successfully recruited, such as the Prostate Testing for Cancer and Treatment (ProtecT) study (www.nets.nihr.ac.uk/projects/hta/962099). This trial more than doubled its recruitment rate by employing a QuinteT recruitment investigation (QRI) run by the University of Bristol School of Social and Community Medicine. Hence, we will also use Bristol’s QRI methodology in the TRoMbone feasibility study to understand the recruitment process and how it will operate in each of the three centres, so that sources of recruitment difficulties can be identified and suggestions made to change aspects of design, conduct, organisation or training that could then lead to improved recruitment rates. Should successful randomisation be demonstrated in TRoMbone, this would rationalise a larger, clinical trial focused on oncological outcomes.
TRoMbone has been accepted onto the National Institute of Health Research Portfolio and thus has access to Clinical Research Network support. It has ethical and other regulatory approvals, and opened in February 2017. We have 12 months to recruit the 50 patients, and if we miss this target it is highly unlikely to proceed to a full study. Thus, we are asking the UK urological community to refer eligible patients to one of the three sites. Centres that demonstrate the ability to identify and refer eligible patients will be taken forward in the full grant application if feasibility is demonstrated. Patients can start systemic therapy as part of normal care prior to referral, so there is no delay in their treatment and no increased risk of breaching cancer waiting time targets. Those randomised to surgery will have their operations done at the referral centres as well as a single follow-up visit before discharge back to the local referring unit; participants randomised to systemic therapy alone will require just one follow-up visit with the referral centre. Hence, the extra travel burden on study patients from local centres is minimal. TRoMbone represents a real opportunity for the urological community in the UK to conduct a study with global impact and the potential to transform the management of early, lethal prostate cancer. We hope you can be a part of it and together we can make it a success!
Acknowledgements
TRoMbone is funded by awards from the Prostate Cancer Foundation and The Urology Foundation.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67(1):7-30.
2. James ND, et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the "Docetaxel Era": Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019). Eur Urol 2015;67(6):1028-38.
3. Sweeney CJ, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med 2015;373(8):737-46.
4. Stevens DJ, Sooriakumaran P. Oligometastatic Prostate Cancer. Curr Treat Options Oncol 2016;17(12):62.
5. Ghadjar P, et al. The oncologic role of local treatment in primary metastatic prostate cancer. World J Urol 2015;33(6):755-61.
6. Wiegand KC, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 2011;224(3):328-33.
7. Won AC, et al. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int 2013;112(4):E250-5.
8. Weckermann D, et al. Perioperative activation of disseminated tumor cells in bone marrow of patients with prostate cancer. J Clin Oncol 2009;27(10):1549-56.
9. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol 2014;65(6): 1058-66.
10. Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. Eur Urol 2014;66(3):602-3.
11. Thompson IM, et al. Impact of previous local treatment for prostate cancer on subsequent metastatic disease. J Urol 2002;168(3):1008-12.
12. Swanson GP, Riggs M, Earle J. Failure after primary radiation or surgery for prostate cancer: differences in response to androgen ablation. J Urol 2004;172(2):525-8.
13. Shao YH, et al. Cancer-specific survival after metastasis following primary radical prostatectomy compared with radiation therapy in prostate cancer patients: results of a population-based, propensity score-matched analysis. Eur Urol 2014;65(4):693-700.
14. Bayne CE, et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. Eur Urol 2016;69(5):775-87.
15. Sooriakumaran P, et al. A Multi-institutional Analysis of Perioperative Outcomes in 106 Men Who Underwent Radical Prostatectomy for Distant Metastatic Prostate Cancer at Presentation. Eur Urol 2016;69(5):788-94.
16. Tewari A, et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 2012;62(1):1-15.
17. Fossati N, et al. Identifying optimal candidates for local treatment of the primary tumor among patients diagnosed with metastatic prostate cancer: a SEER-based study. Eur Urol 2015;67(1):3-6.
18. Decaestecker K, et al. Surveillance or metastasis-directed Therapy for OligoMetastatic Prostate cancer recurrence (STOMP): study protocol for a randomized phase II trial. BMC Cancer 2014;14:671.
19. Tree AC, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14(1):e28-37.
20. Treasure T, et al. Pulmonary metastasectomy in colorectal cancer: the PulMiCC trial. Thorax 2012;67(2):185-7.




