Renal calculi can be managed according to four treatment options: conservative management, extracorporeal shockwave lithotripsy (ESWL), flexible ureterorenoscopy (FURS) and percutaneous nephrolithotomy (PCNL).
This is the first in a two-part series in Urology News (Part 2 available here) that will address conservative management and ESWL (this article) with FURS and PCNL to be covered in a future issue. Each modality of treatment has been divided into:
- Patient selection (including ‘the perfect case’ and ‘the case to avoid’)
- Intra-treatment decision-making
- Postoperative management / follow-up
Conservative management of renal stones
Patient selection
The management of the asymptomatic, small renal stone draws some parallels with low grade, low volume prostate cancer. Before embarking upon the treatment of any stone, a collection of factors has to be considered (some of which are outlined in Table 1). Patient engagement in the treatment path is crucial: this is particularly pertinent when considering conservative management which may be considered as the endourological form of ‘active surveillance’. In this regard, ‘progression’ could be used as a term to indicate that the patient has either developed symptoms or whose stone burden has increased in size such that intervention may be required.
The prevalence of the asymptomatic stone is quoted within the region of 8-10% of the population with an average diameter of stone at approximately 3mm [1,2]. Stone size is a key variable in the failure of observation and the subsequent need for future intervention. Koh and colleagues found that smaller stones (average size 5.7mm), had a greater likelihood of spontaneous passage (20%) with a low incidence of intervention (7.1%) [3].
The position of the stone within the collecting system can often come with some treatment dilemmas. In particular, the lower pole stone often has all treatment options and modalities available. Yuruk et al. [4] noted more than 20% of patients with lower pole stones <20mm required some stone-related intervention. However, Keeley et al. [5] found no difference in the outcome of the management of lower pole stones <15mm between ESWL and ‘active surveillance’. In general, patients with asymptomatic stones should be counselled that, although approximately 50% will progress, most of these will not require surgery. Outcomes of asymptomatic stones <10mm in diameter include a symptomatic stone event (13-32%), spontaneous passage (13-20%), size increase (30-46%) and intervention (7-26%) [6].
The perfect case
The ideal case would be a small asymptomatic stone ≤5mm that has been stable in size for some years, and which is located in a ‘non-threatening position’ which does not risk impending renal obstruction, and which could be treated easily with all options available should the stone progress.
The cases to avoid
Conservative management is not advised in cases where there is poor compliance to follow-up or the rate of increase to the patient’s stone burden is uncontrolled. Factors that have been associated with failed observation include: ≥6mm on presentation, lower calyceal stones (more likely to grow), and high urine osmolality [7]. The larger the stone, the greater the chance of progression: 100% of stones greater than 15mm within the renal pelvis progress [7]. Assuming treatment is going to be offered at some stage, such patients should be offered active intervention from the outset, rather than waiting and increasing the likelihood of needing a PCNL rather than FURS, and also of leaving residual fragments that might cause future problems in their own right (see the second part of the article for details).
Patients with a solitary anatomical or functioning kidney (due to the consequences of more invasive future management if progression occurs), those with urinary tract reconstruction (where emergency access from below may be challenging if acute colic / obstruction occurs) or with immunodeficiency (in whom the morbidity of infection is a concern) may not be appropriate for observation, even if they are asymptomatic. Conservative management is also not advised in children, who are more likely to have an underlying metabolic abnormality, and therefore to progress, as well as being better managed electively than in an emergency presentation with pain and / or sepsis.
The occupation of the patient will occasionally play a part in the management recommendations provided. The UK Civil Aviation Authority (CAA) is the only civilian body that has formalised guidelines for the management of urinary stone disease. The Royal Airforce grounds any aircrew on the discovery of a renal calculus. Residual stones result in continued grounding and a metabolic investigation is required within six weeks of the first stone episode for all personnel. Similarly, undersea divers in the Royal Navy are rejected for service unless their stones have been adequately treated [8]. Staghorn calculi should generally be treated actively from the outset: seminal papers from Singh et al. [9] and Teichman et al. [10] have demonstrated a 50% chance of nephrectomy and a 67% chance of renal-related mortality if staghorn stones are left untreated.
Follow-up
Follow-up regimes for asymptomatic renal calculi can often require a nuanced approach, where the multiple factors above have to be balanced, and an assessment of risk / likelihood of progression made to guide the timing and duration of follow-up, with patient ‘buy-in’ to the recommendation clearly essential.
At its simplest, patients with smaller stones identified incidentally can be discharged back to the general practitioner for consideration of a follow-up in a year’s time with urinalysis, creatinine and either an ultrasound or x-ray KUB (if radio-opaque) – with the understanding of a re-referral if stone size progressively increases or if the patient becomes symptomatic. At the other end of the scale, high-risk stone formers (i.e. patients with anatomical abnormalities / genetic predisposition or diseases associated with stone formation) should be kept on the auspices of the urology department – with follow-up ranging from six months to a year according to the European Association of Urology (EAU) Guidelines [11]. These patients might also benefit from a formal metabolic assessment to identify reversible / treatable causes and allow a more tailor-made preventative approach against progression of any untreated stones.
Extra corporeal shockwave lithotripsy (ESWL)
The use of ESWL for renal stones has taken a back-seat due to the rise of endourological treatments for stones over the last decade, perhaps as a consequence of fewer advances in lithotripter technology compared with those in flexible ureterorenoscopy (digital scopes, including single-use devices), combined with improving strategies to reduce morbidity related to postoperative JJ stents (e.g. overnight ureteric catheters or stents on strings). Despite this, appropriately targeted ESWL (both to the patient and to their stone) remains a valuable tool in the management of renal stone disease.
Patient selection
Often described as similar to the sensation of ‘a stretched rubber-band striking the skin repetitively’, ESWL is a treatment modality that is generally well accepted by patients due to its least invasive approach, treatment duration (20-30 minutes) and its delivery in an outpatient setting. Notwithstanding its favourability with patients and low-risk profile, precise patient selection improves success rates.
Patient selection requires an assessment of the pelvicalyceal anatomy, stone density on imaging and stone size: the EAU guidelines state that ESWL achieves good stone-free rates (SFR) for stones up to 20mm, except for those at the lower pole. For stones >20mm ESWL is likely to require multiple treatments, carries an increased risk of ureteric obstruction (either colic or Steinstrasse) and an increased likelihood of requiring adjunctive procedures [11].
In their systematic review comparing ESWL vs. retrograde intrarenal surgery (RIRS) vs. PCNL for the treatment of lower pole stones ≤20mm in adults, Donaldson et al. analysed seven randomised controlled trials (RCT) involving 691 patients and demonstrated that the likelihood of being stone free within three months was twice as good for PCNL over ESWL (risk ratio: 2.04; 95% CI 1.50-2.77), but the benefit was markedly less for ≤10mm stones [12].
These conclusions are supported by a Cochrane Review [13] assessing the use of ESWL compared to PCNL or RIRS for kidney stone management, which found that ESWL is less effective for kidney stones than PCNL but not significantly different from RIRS. Individual episodes including hospital stay and duration of treatment were shorter with ESWL at the expense of a more frequent re-treatment rate. Auxiliary procedures were more commonly associated with ESWL although the overall success of treatment was not significantly different at the end of the third month. Mean procedural time and mean hospital stay was reported to be longer in the RIRS group.
A large body mass index (BMI>30) [14], has a negative correlation with the stone-free rate due to the longer skin-to-stone distance (SSD). Patients with complex intra- or extra-renal anatomy (e.g. chronic pelvi-ureteric junction (PUJ) obstruction or horseshoe configuration) also fare less favourably when it comes to expelling stones once fragmented as a consequence of impaired drainage.
Patient compliance is important to the success of ESWL – the clinician will need to ensure the patient accepts the need to attend for a number of sessions over a period of several weeks, with no guarantee of complete stone clearance.
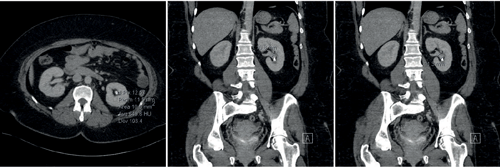
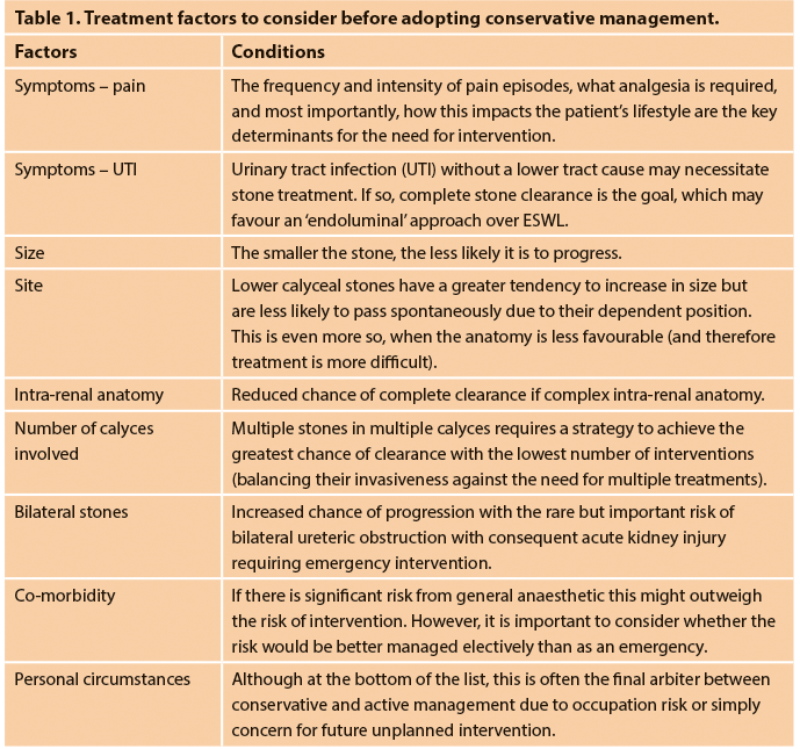
Figure 1a. 650HU, lower pole stone with a wide (11mm) and short (11mm) infundibulum.
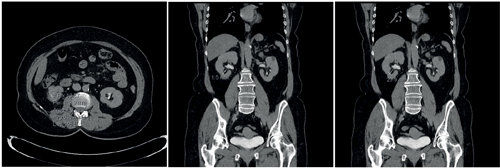
Figure 1b. 635HU, lower pole stone with a narrow (1.5mm) and long (21mm) infundibulum.
The perfect case
The maximum SSD varies according to the lithotripter in use and its specific focal length; but studies generally point to an SSD<10cm as appropriate. Although ESWL can treat (i.e. fragment) stones in all calyces, those within the lower pole have the lowest rate of clearance (25%). The intra-renal anatomy affects this further, with a wide (>5mm), short (<10mm) infundibulum, with a shallow infundibulo-pelvic angle (>70o) allowing better clearance of fragments than if the stone is at the end of a tight, long infundibulum.
Hounsfield unit density can be used as a guide to the stone’s ‘hardness’, with various studies giving different cut-off values for predicting success. Hard stones (calcium oxalate monohydrate, or the ‘Brushite’ form of calcium phosphate stones) fragment less readily and therefore are more likely to cause obstruction on travel or indeed may not fragment at all. Ouzaid et al. evaluated the outcome four weeks after ESWL by non-contrast computed tomography (NCCT) and found that patients whose stones had a Hounsfield unit density <970 had a stone-free rate (including “clinically insignificant residual fragments ≤4mm”) of 96%, but this was just 38% if the stones HU density was ≥970HU (P<0.001) [15]. Furthermore, various parameters can be combined to give even more precise prediction of stone-free rates: using a combination of stone density <600HU, skin-to-stone distance <12cm and stone volume, Tran et al. demonstrated a likelihood of being stone free increased from 21.4% (if all parameters were unfavourable) to 96.1% if all were favourable [16].
Ultimately, size remains the key determinant of successful SWL, such that stones less than 1cm are preferred due to the likely size and number of the subsequent residual fragments. Although several papers discuss the options of ESWL in stone sizes up to 2cm (this is similarly stated within the EAU Guidelines), in reality stones of these sizes are usually treated by an endourological approach.
The case to avoid
ESWL should be avoided in patients who are pregnant, mandatorily anti-coagulated, with uncontrollable urinary tract infections, and considered with caution in patients with aortic or renal artery aneurysms. Severe skeletal abnormality is not an absolute contraindication and can be utilised if there is a ‘window’ whereby the acoustic waves can be focused on the stone. However, the anatomical contortion carries a challenge for adequate coupling, along with the potential for dissipation of the shockwave energy which can impact on the effectiveness of the treatment. It can also create issues for pain control and can be damaging to non-renal structures. The presence of a pacemaker / defibrillator is also not an absolute contra-indication to ESWL (details must be cross-checked against the specific manufacturer), although many units are reticent to proceed and will therefore recommend an endourological approach for treatment instead.
As is often mistakenly stated to patients, stents do not ‘help stone fragments to pass’ – this has been confirmed in a systematic review on the use of the ureteric stent alongside ESWL which showed no added advantage in the stone-free rate between the stented and unstented cohorts, but did show a significant exacerbation of lower urinary tract symptoms (LUTS) from stent symptoms [17]. Patents who have been stented as an emergency are therefore best advised to proceed directly to URS (facilitated with the passive ureteric dilatation from the stent) to prevent protracted stent symptoms and increased risk of infection if the dwell time is too long.
Figure 2. Stone visible on scout film – for intraoperative targeting and follow-up.

Figure 3. Patient performing percussion, diuresis and inversion at home.
Intra-treatment decision-making
Although ESWL is contraindicated in an untreated infection, there is no level 1 evidence in favour of antibiotic prophylaxis in ‘routine’ cases, which therefore varies from unit to unit. In our unit, if the patient is asymptomatic, treatment is delivered without any antibiotics; if there are symptoms suggestive of a UTI and a dipstick analysis is positive for nitrites, a midstream specimen of urine (MSU) is sent, antibiotics are started, and the ESWL session is deferred.
Adequate analgesia pre-procedure will help to mitigate pain and allow the full procedure to take place at an appropriate shockwave intensity. If the patient struggles to tolerate the treatment despite reducing the rate and strength of the shockwaves, varying the patient’s position in relation to the lithotripter head can occasionally alleviate discomfort. Voltage ramping can also be useful to ‘ease’ the patient into the procedure, and minimise discomfort, particularly the surprise effect of the very first shock delivered. Furthermore, voltage ramping has been shown to improve stone fragmentation as well as having a protective effect on the kidney [18]. This technique delivers <100 shocks to the kidney at a lower energy setting, before gradually increasing the power. This acts to:
- Improve tolerance to shocks (less pain).
- Improve fragmentation rates – starting at low power prevents build-up of smaller stones in front of target thereby attenuating shock strength.
- Reduce renal damage by increasing the resistive index of renal vasculature due to vasoconstriction (and consequently less risk of bleeding).
Reducing the rate of shockwave delivery from 2Hz to 1Hz also improves stone fragmentation (by optimising bubble cavitation on stone surface) but obviously increases the duration of the procedure [19].
Postoperative management
The complications from ESWL can be divided into those arising from the formation and passage of fragments, post-procedure infection and the effects of shockwaves on tissue and on renal function. Whilst the risk of complications from the passage of fragments can be minimised by appropriate patient selection as described above (i.e. avoiding stones that are too large / hard), the effects of shockwaves on tissue and renal function are less dependent on stone-related factors.
The prevalence of peri-renal haematoma is quoted as around 13% [20], with symptoms less common at between 4-6% [21]. The risks are dependent on the skill of the technician and patient factors including hypertension, higher BMI and larger stone size, but low-dose aspirin does not seem to affect risk [22]. The relative risk increases by a factor of 1.67 for each 10-year incremental age increase [23]. Fortunately, the incidence of transfusion is negligible; Lee et al. quote a risk of 0.04% (just four patients needed a transfusion in their series of over 10,000 patients) [24].
‘Percussion, diuresis and inversion’ (PDI), may be suggested to aid the passage of the lower pole stone fragments. In the Donaldson study [13], RIRS had a greater likelihood of achieving stone-free over SWL (risk ratio 1.31; 95% CI, 1.08-1.59), but this can be reduced with auxiliary manoeuvres such as PDI therapy [25].
Stone-free rates are suggested to range from 40-60% [26], depending on the methods used. Diuresis can be stimulated by a 500ml bolus of water or 20mg of furosemide, whilst inversion (of 45 degrees) and percussion are usually performed in the comfort of the patient’s home surroundings (Figure 3). Not surprisingly, this cannot always be feasible and therefore a suggested modified approach includes swimming after ingestion of 500ml of water.
The modality of choice for follow-up imaging should correspond with the pre-procedural decision-making and goal of treatment. Whilst KUB x-ray and renal tract ultrasound are the most common modalities used in follow-up to assess for residual stones that may benefit from a repeated course of SWL treatment. Patients who need a genuinely stone-free kidney should have a low-dose non-contrast CTKUB to ascertain the exact post-treatment stone status. Certainly, if more invasive intervention is considered for ‘ESWL failure’ a CT will be needed to plan endourological ‘salvage’ treatment appropriately.
Conclusion
Treatment decisions for stone-bearing patients are complex and multifactorial, based on the combination of stone factors, their intra-renal anatomy and down-stream drainage, and ‘the whole of the rest of the patient’ i.e. their co-morbidity, social circumstances and expectations for treatment. We hope this article has put some of these issues into context for the more conservative options for stone management, and will explore these in similar manner for FURS and PCNL in the next article in this series.
References
1. Boyce CJ, Pickhardt PJ, Lawrence, et al. Prevalence of urolithiasis in asymptomatic adults: objective determination using low dose noncontrast computerized tomography. J Urol 2010;183(3):1017-21
2. Lorenz EC, Lieske JC, Vrtiska TJ, et al. Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant 2011;26(8):2695-700.
3. Koh LT, Ng FC, Ng KK. Outcomes of long-term follow-up of patients with conservative management of asymptomatic renal calculi. BJU Int 2012;109(4):622-5.
4. Yuruk E, Binbay M, Sari E, et al. A prospective, randomized trial of management for asymptomatic lower pole calculi. J Urol 2010;183(4):1424-8.
5. Keeley FX Jr, Tilling K, Elves A, et al. Preliminary results of a randomized controlled trial of prophylactic shock wave lithotripsy for small asymptomatic renal calyceal stones. BJU Int 2001;87(1):1-8.
6. Goldsmith ZG, Lipkin ME. When (and how) to surgically treat asymptomatic renal stones. Nat Rev Urol 2012;9(6):315-20.
7. Burgher A, Beman M, Holtzman JL, Monga M. Progression of nephrolithiasis: long-term outcomes with observation of asymptomatic calculi. J Endourol 2004;18(6):534-9.
8. Borley NC, Rainford D, Anson KM, et al. What activities are safe with kidney stones? A review of occupational and travel advice in the UK. BJU Int 2007;99(3):494-6.
9. Singh M, Chapman R, Tresidder GC, et al. The fate of the un-operated staghorn calculus. Br J Urol 1973;45(6):581-5.
10. Teichman JM, Long RD, Hulbert JC. Long-term renal fate and prognosis after staghorn calculus management. J Urol 1995;153(5):1403-7.
11. Türk C, Neisius A, Petrik A, et al. EAU Guidelines in Urolithiasis. 2016.
http://uroweb.org/guideline/urolithiasis/
12. Donaldson JF, Lardas M, Scrimgeour D, et al. Systematic review and meta-analysis of the clinical effectiveness of shock wave lithotripsy, retrograde intrarenal surgery, and percutaneous nephrolithotomy for lower-pole renal stones. Eur Urol 2015;67(4):612-16.
13. Srisubat A, Potisat S, Lojanapiwat B, et al. Extracorporeal shock wave lithotripsy (ESWL) versus percutaneous nephrolithotomy (PCNL) or retrograde intrarenal surgery (RIRS) for kidney stones. Cochrane Database Syst Rev 2014;24(11):CD007044.
14. Pareek G, Armenakas NA, Panagopoulos G, et al. Extracorporeal shock wave lithotripsy success based on body mass index and Hounsfield units. Urology 2005;65(1):33-6.
15. Ouzaid I, Al-qahtani S, Dominique S, et al. A 970 Hounsfield units (HU) threshold of kidney stone density on non-contrast computed tomography (NCCT) improves patients’ selection for extracorporeal shockwave lithotripsy (ESWL): evidence from a prospective study. BJU Int 2012;110(11 Pt B):E438-42.
16. Tran TY, McGillen K, Cone EB, Pareek G. Triple D Score is a reportable predictor of shockwave lithotripsy stone-free rates. J Endourol 2015;29(2):226-30.
17. Shen P, Jiang M, Yang J, et al. Use of ureteral stent in extracorporeal shock wave lithotripsy for upper urinary calculi: a systematic review and meta-analysis. J Urol 2011;186(4):1328-35
18. Wollin DA, Preminger GM. Percutaneous nephrolithotomy: complications and how to deal with them. Urolithiasis 2018;46(1):87-97.
19. Madbouly K, El-Tiraifi AM, Seida M, et al. Slow versus fast shock wave lithotripsy rate for urolithiasis: a prospective randomized study. J Urol 2005;173(1):127-30.
20. Skuginna V, Nguyen DP, Seiler R, et al. Does stepwise voltage ramping protect the kidney from injury during extracorporeal shockwave lithotripsy? Results of a prospective randomized trial. Eur Urol 2016;69(2):267-73.
21. Orozco Fariñas R, Iglesias Prieto J, Massarrah Halabi J, et al. [Renal hematoma after extracorporeal shockwave lithotripsy in a series of 324 consecutive sessions with the DOLI-S lithotripter: incidents, characteristrics, multifactorial analysis and review]. Arch Esp Urol 2008;61(8):889-914.
22. Schnabel MJ, Gierth M, Chaussy CG, et al. Incidence and risk factors of renal hematoma: a prospective study of 1,300 SWL treatments. Urolithiasis 2014;42(3):247-53.
23. Dhar NB, Thornton J, Karafa MT, Streem SB. A multivariate analysis of risk factors associated with subcapsular hematoma formation following electromagnetic shock wave lithotripsy. J Urol 2004;172(6 Pt 1):2271-4.
24. Lee HY, Yang YH, Shen JT, et al. Risk factors survey for extracorporeal shockwave lithotripsy-induced renal hematoma. Endourol 2013;27(6):763-7.
25. Lee SW, Chaiyakunapruk N, Chong HY, Liong ML. Comparative effectiveness and safety of various treatment procedures for lower pole renal calculi: a systematic review and network meta-analysis. BJU Int 2015;116(2):252-64.
26. Chiong E, Hwee ST, Kay LM, et al. Randomized controlled study of mechanical percussion, diuresis, and inversion therapy to assist passage of lower pole renal calculi after shock wave lithotripsy. Urology 2005;65(6):1070-4.
Declaration of competing interests: None declared.