In 2018 the United Kingdom Government spending on healthcare totalled almost £166 billion. Of this approximately 65% was attributed to providing curative or rehabilitation therapy, with health-related long-term care and provision of goods accounting for 25%. The remaining was accredited to preventative care and healthcare governance. Including both government and non-government spending this equated to £3200 per person.
The Global COVID-19 pandemic resulted in an unprecedented demand for healthcare services that has required an additional injection of funding.
The increasing cost of healthcare, a need to address the inevitable case load backlog that has arisen, and the anticipated shortfall in healthcare staff has increased the importance of innovation in healthcare services.
From a clinical perspective the primary aim of medical innovation is that of improving patient care. Secondary rewards include academic interest and the potential to undertake research. Additional benefits include improving the status of a clinical unit and with that the potential to attract a greater case load.
The recent rebranding of NHS Research and Development departments into Research and Innovation departments has demonstrated a clear statement of intent. Hospital trusts are conscious of the potential cost saving of a successful process but also the financial return of a successful product. There is often a contractual agreement between a clinician and their employer to declare an innovation and potentially share the intellectual property rights and profits derived from that project. We would hence encourage NHS staff to register their innovation early during the process and establish the extent of support that will be provided. This is essential as some trusts may demand over 75% of the net profits generated by an innovation, despite neither funding nor allowing the innovator time to develop their product.
What is medical innovation?
A new process, product, service, method or technology may be considered to be ‘innovative’, and when applied to healthcare a ‘medical innovation’.
Clinical medicine often requires adapting one’s approach to a patient, surgical technique, and on occasion modifying a device. In the case of the latter, this may result in a novel medical innovation.
Innovations may be truly novel (e.g. recording of the electrocardiogram by Augustus Waller, or the discovery of penicillin by Alexander Fleming), adaptation of an existing device (e.g. bone anchored hearing aids and dental implantation), or the application of an existing product in a different surgical field (e.g. the microdebrider in both orthopaedic surgery and urology).
The innovation process
An understanding of the innovation pathway is key to navigating a project to a successful conclusion. This is outlined in Figure 1.
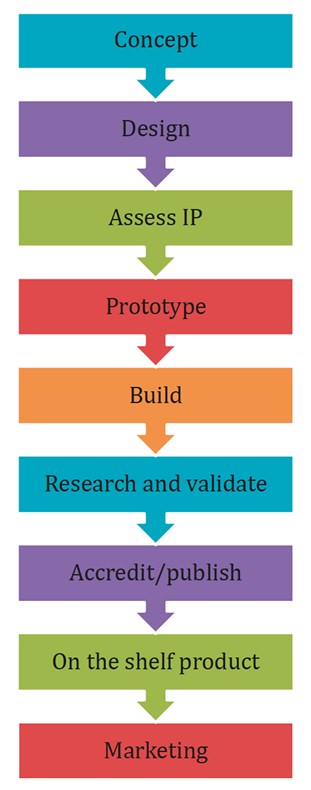
Figure 1: The innovation pathway.
Concept
Successful projects arise from first identifying a clinical need. On occasion clinicians with relatively little experience provide a solution for a problem that does not exist, or may provide little or no benefit over an existing product or pathway. New innovators should hence be cautious, but their novel perspective may expose a genuinely new product or pathway. If there is doubt about the validity of a project advice should be sought from an experienced team. Once a need has been identified and a solution found this should be acted upon.
When for example an ENT department’s balance assessment equipment was found to be beyond repair and too expensive to replace, an iPhone application D+R Balance, was generated. This App records rotation on Fukuda step testing and postural sway on Romberg’s Testing. The platform required appropriate validation and CE approval via the Medicines and Healthcare products Regulatory Authority (MHRA) [1,2] and is used internationally to assess dizzy patients.
Design
A good design should be simple and clearly resolve the clinical problem that it addresses. Complex designs or designs that set out to solve multiple problems should be avoided if possible.
Assess intellectual property (IP)
Is your idea truly novel? It is worthwhile spending time researching patents, publications and the internet. It is often useful to hire a specialist in this field as some patents may be difficult to find.
Prototype
Innovators are always encouraged to fashion a prototype themselves. This allows them to gain an understanding of the materials that may be used and the parts required to develop a working model. For example, a piezoelectric plate soldered to a 3.5mm audio jack will provide an acoustic stimulus.
The iPhone, D+R Hearing, replaced standard tuning fork testing and assessed bone conductor thresholds. Unfortunately, a smartphone shift to Bluetooth headsets made this project unfeasible and it was therefore suspended. However, adaptation of this process by including a signal return recording and attachment above the pubis, may provide a simple and inexpensive bladder filling sensor that notifies a carer of when a patient may need to void.
Build
It is often the case that a physical build will require working with an established company. One should always seek a non-disclosure agreement (NDA) when approaching potential collaborators and we would encourage innovators to keep their ideas private and not to present them at meetings nor publish them on the internet.
Some projects can be built with a determined team. For example, Docbook.co.uk was an online picture book series that Dr Aslan Mirza, a GP with formidable IT skills, Dr Junaid Bajwa (now Chief Medical Scientist for Microsoft) and Rahul Kanegaonkar published in 2007. This online picture book series attracted over 3.2 million hits from over 150 countries.
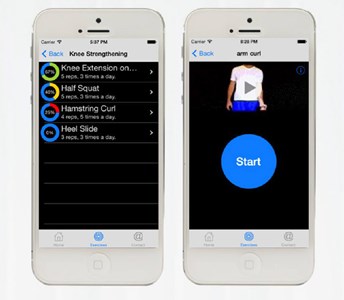
Figure 2: The Flexio platform, provides remote monitoring and
supervision of patients undergoing prehabilitation programmes
and may be of benefit in patients undergoing robotic prostatectomy.
More complex digital platforms require an understanding of security and regional protocols. Flexio, a website / smartphone interface, allows the remote supervision of patients undergoing musculoskeletal physiotherapy (Figure 2). This not only improves compliance but also reduces the potential need for weekly review [3,4]. The cost saving to the NHS is potentially enormous. Adapted, this could be used to provide prehabilitation exercises for patients undergoing robotic prostatectomy.
Research and validation
Some but not all innovation projects will require a form of validation. The Cupris iPhone Otoscope, for example, provides an elegant remote method for assessing and recording ear pathology that was validated by our team. A simple lens attachment converts the smartphone into a digital otoscope that can be used as a screening tool for potential referrals.
The Digital Ear Trainer developed by Adam Rouilly was an interesting validation project. This teaching aid was tested and the data used to support this simulator’s penetration into the market.
Accredit / publish
Clinicians are by nature risk averse. Many will be more comfortable using a new innovation having assessed the evidence base. Hence, peer reviewed publication is on occasion a necessity.
On the shelf product and marketing
Marketing of a novel innovation can require substantial investment. Advertising in medical journals and via specialist marketing companies may be essential to get the word out.
The landscape for medical innovation has changed enormously over recent years and more so due to the COVID-19 pandemic. Several platforms have been launched offering guidance (e.g. the Institute of Medical Sciences Innovation Hub at Canterbury Christ Church University). Innovators must have a clear understanding of the role of the platform, the services they provide and their cost, both in the initial phase and downstream.
The Clinical Entrepreneurship Programme, launched by Professor Tony Young, the National Clinical Director for Innovation at NHS England, is an extraordinary platform that provides tuition and networking opportunities. Successful applicants are exposed to tutorials that cover such topics as how to pitch, marketing and raising funds.
Cross specialty innovation – urology
With a near exponential increase in healthcare innovation, application and uptake, the future will usher in a new paradigm in the development of innovative platforms. Urology, in particular, has been at the forefront of medical technology and innovation. Over the last three decades, the specialty has seen a rapid evolution in endourological tools and techniques including the use of laser technology and robot assisted surgical platforms. Transoceanic telerobotic surgery has been successfully performed, demonstrating the potential of advanced innovations from concept to delivery. Innovations further pushed the boundaries of technology as demonstrated by the successful application of laparoendoscopic single site surgery and successful use of surgical mapping and assist tools such as virtual augmented reality [5].
The landscape continues to evolve with novel concepts and tools being developed such as large-scale data fusion for research purposes, biomarkers, intelligent probes [6], wearable remote monitoring devices and nanotechnology [7]. Artificial intelligence and machine learning tools will continue to be applied to urological diagnostics and therapeutics in the near future. The rapid evolution of innovation in healthcare propels individual healthcare providers and scientists to explore the need for the implementation of various targeted curriculums within existing subspecialties.
Conclusion
We would certainly encourage clinicians at all stages of their careers to consider developing their medical innovation projects. Some projects may fail, and we often remind innovators that their first innovation may not be their best, but that they will come to understand the process and avoid pitfalls with future projects. A minority may prove lucrative but they will all be interesting and challenging.
References
1. Whittaker M, Mathew A, Kanani R, Kanegaonkar RG. Assessing the Unterberger test: introduction of a novel smartphone application. J Laryngol Otol 2014;128(11):958-60.
2. Yvon C, Najuko-Mafemera A, Kanegaonkar R. The D+R Balance application: a novel method of assessing postural sway. J Laryngol Otol 2015;129(8):773-8.
3. Vaish A, Ahmed S, Shetty A. Remote physiotherapy monitoring using the novel D+R Therapy iPhone application. J Clin Orthop Trauma 2017;8(1):21-4.
4. Saleh A, Parkar F, Tolat A. Management of Radial Head Fracture. Res Rev Orthop 2018;2(1).
5. Rane A, Ahmed S, Kommu SS, et al. Single-port ‘scarless’ laparoscopic nephrectomies: The United Kingdom experience. BJU Int 2009;104(2):230-3.
6. Kommu SS , Andrews RJ, Mah RW. The future role of intelligent probes in detecting and managing prostate cancer. BJU Int 2006;98(4):717-19.
7. Tewari A (Ed.). Prostate Cancer: A Comprehensive Perspective. London, UK; Springer-Verlag; 2013.
Declaration of Competing Interests: Rahul Kanegaonkar is a Director of D+R Medical Limited that developed the D+R Therapy Platform.