Enhanced recovery after surgery (ERAS) protocols are patient pathways designed to reduce surgical stress and accelerate postoperative recovery. Uptake of such protocols in colorectal surgery in particular has been high, however ERAS protocols developed or utilised in urological surgery have had a low adoption rate [1].
Radical cystectomy is a significant surgical challenge, and it is associated with greater morbidity and prolonged inpatient stay after surgery than other urological procedures. Despite improvements in anaesthesia and perioperative care over the past decade, the morbidity associated with radical cystectomy remains above 30% [2]. Cystectomy patients may therefore be ideal candidates for an ERAS pathway, as the potential for reduction of surgical stress and complications is high.
The presented review discusses particular aspects of an ERAS protocol for robotic cystectomy patients, looking in particular at new techniques in preoperative assessment including cardio-pulmonary exercise testing (CPET), preoperative nutrition, intraoperative fluid optimisation with oesophageal Doppler, the impact of a minimally invasive surgical technique, and postoperative interventions to reduce ileus.
Introduction
Enhanced recovery is a multidisciplinary approach to the entire patient perioperative pathway that is designed specifically to optimise patient care at each and every stage of the patient journey for a specific operative procedure. The intention of this bundle of interventions being that patients will recover faster from surgery, with a reduction in complication rate and a shorter hospital stay.
The concept was first pioneered by a Danish surgical professor Henry Kehlet in the 1990s and has since been rolled out across many surgical specialties, creating procedure specific ERAS protocols in the fields of colorectal, urological, gynaecological, vascular and orthopaedic surgery. These ERAS protocols are delivered by a team of professionals – anaesthetists, surgeons, physiotherapists, nurses – and are designed to modify the physiological and psychological response to the stress of major surgery.
There are four key strands to any ERAS protocol:
- Appropriate preoperative assessment, patient identification and preparation prior to admission.
- Reducing physical stress of the operation – through a series of modifications to surgical and anaesthetic intraoperative care.
- A structured approach to the immediate postoperative care, including pain relief and nutrition.
- Early mobilisation.
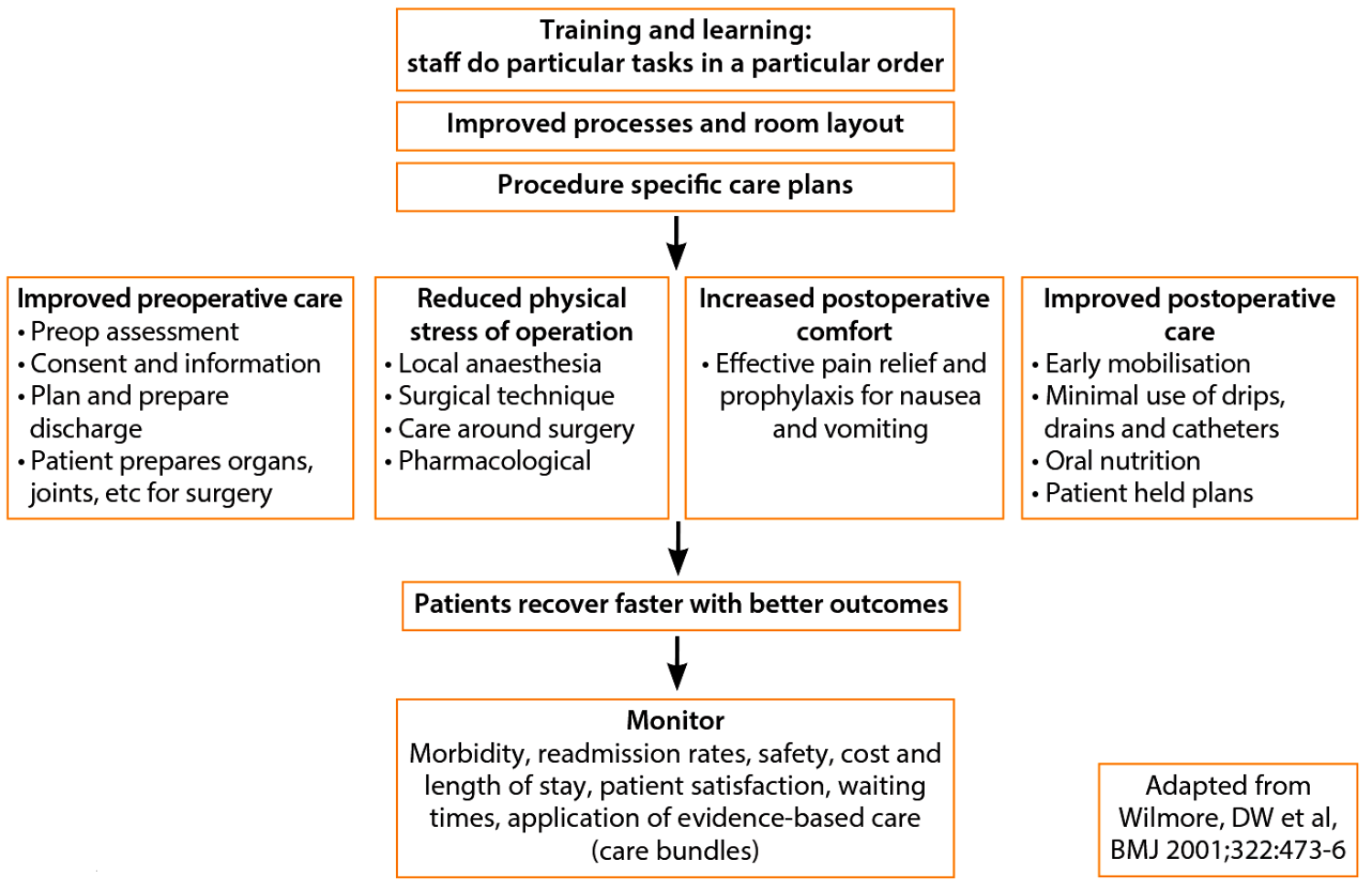
Figure 1.
Figure 1, adapted from Wilmore et al., illustrates that initiating a procedure-specific ERAS protocol needs a systematic, multi-disciplinary approach with robust standardised protocols incorporating audit of practice to ensure adherence to the ERAS system and monitoring of outcomes.
There is a nationwide ERAS network within the UK, and the ERAS society – an international society founded in Sweden in 2011 – holds its 2nd World Congress on Enhanced Recovery in November 2014. This demonstrates that widespread adoption of ERAS protocols and the ERAS concept is high on the agenda internationally. The ERAS society has recently published guidelines specific to major urological surgery: radical cystectomy [1]. Their review analyses the current evidence base for ERAS interventions specific to cystectomy, and also assesses if protocols in place for colorectal surgery could be extrapolated to cystectomy.
Robotic radical cystectomy
Radical cystectomy with pelvic lymph node dissection is the gold standard for muscle-invasive bladder carcinoma [3]. It is a major uro-oncological pelvic procedure with many potential complications including postoperative cardiorespiratory failure, deep vein thrombosis, ileus and metabolic derangement. Morbidity after open radical cystectomy with bilateral pelvic node dissection and urinary diversion or bladder reconstruction is 30-64%.
This is thought to be as a result of the significant pro-inflammatory response to radical cystectomy, resulting in a high postoperative oxygen demand and significant fluid shifts in the perioperative period. Robotic radical cystectomy offers a minimally invasive approach, using the latest in surgical and robotic technologies, and is being increasingly used worldwide. Minimally invasive surgery is a key component of any ERAS pathway, as it has been shown to decrease the patients’ inflammatory response when compared to an open approach. In rectal surgery for example, this has been translated into a reduction in postoperative ileus, complications and length of stay.
First reported in 2003 as a feasible approach to radical cystectomy [4], robotic surgery has been adopted worldwide, and has been associated with lower overall perioperative complications and shortened length of stay [5]. It has also been associated with less blood loss, less scarring and less pain from surgery. Robotic surgery however is not without its physiological challenges. It requires a prolonged period of steep Trendlenburg position, together with pneumoperitoneum, which can produce dramatic physiological derangement, particularly in the more elderly populations with multiple co-morbidities who present for cystectomy. It is therefore essential that thorough pre-assessment is undertaken to identify those who are suitable for operative intervention.
Preoperative assessment
At the core of any ERAS protocol is good communication between the patient, urologist, urology stoma nurse specialist, anaesthetist and general practitioner. The pre-anaesthetic assessment clinic is important in identifying patients at high risk of postoperative morbidity, as this will help guide the risk versus benefit ratio of operative intervention, and furthermore determine their postoperative care requirements. Taking a thorough history, and identifying pre-existing cardiovascular and respiratory disease is often done in a nurse-led clinic, where simple self-assessment questionnaires such as the Duke Activity Status Index, can be used to measure a patient’s functional capacity, and get a rough estimate of a patient’s peak oxygen uptake. One metabolic equivalent (MET) represents the oxygen consumption of an adult at rest, i.e. 3.5ml/kg/min. Varying degrees of exercise are designated a number of METs. Patients being considered for major surgery should be able to perform >4 METs, the equivalent exertion of climbing one flight of stairs. Unfortunately many patients overestimate their exercise capabilities, and so more objective assessments are needed.
Other risk scoring systems such as Goldman, Detsky and Lee predict the likelihood of cardiac complications following major surgery, and Arouzollah provided a risk prediction equation for postoperative respiratory complications. However these scoring systems only focus on single organ failure in populations, and do little to enlighten us as to the severity of each contributing risk factor in individual patients. Echocardiography and spirometry are static tests, and do not inform of the patient’s dynamic cardiorespiratory status. Treadmill exercise electrocardiogram (ECG) testing has low sensitivity and specificity in diagnosing coronary artery disease [6]. A more comprehensive, and objective assessment of a patient’s cardiorespiratory function is CPET.
CPET
CPET is the most reliable and objective test for evaluation of a patient’s functional capacity, and has been demonstrated to predict all cause postoperative mortality [7]. The concept was pioneered by Naimark, Wasserman and McIlroy, who in 1963 showed that exercise tests could be used to detect degrees of cardiovascular dysfunction.
It is a non-invasive test, performed with the patient seated on a bicycle ergometer, breathing through a pressure differential pneumotachograph with respiratory gas analysis and monitored by a 12-lead ECG with ST segment analysis, non-invasive arterial blood pressure monitoring and oxygen saturations.
The gas analysers are able to measure breath-breath-breath oxygen consumption (VO2), and carbon dioxide production (VCO2), and therefore generate the respiratory exchange ratio (RER), the ratio of carbon dioxide produced to oxygen consumed. Lung function in the form of spirometry can also be measured by the pneumotachograph, providing information on gas flows and volumes.
Baseline measurements are obtained for patients at rest seated on the bicycle ergometers, and then through a period of graded exercise, with increasing load on the cycle; the computer records over 5000 measurements including work rate, peak oxygen consumption (peak VO2), VCO2, RER, and anaerobic threshold, and the cardiovascular parameters listed earlier. Computer packages calculate expected normal physiological variables for age, sex, height and weight for comparison.
Readings are classically displayed graphically in a nine-panel plot, showing all the relevant results on one piece of paper.
Underlying physiology
As work rate increases, exercising muscle requires more oxygen to generate adequate levels of adenosine triphosphate (ATP) to maintain muscle activity. This increase in oxygen consumption must be met by increasing oxygen delivery to the tissues, through increasing cardiac output. Oxygen consumption (VO2) is therefore equal to cardiac output (heart rate x stroke volume) multiplied by the arterial-mixed venous oxygen difference: VO2=COxC(a-v)O2
It is therefore clear that CPET tests ventricular function, in the form of cardiac output, pulmonary function in the form of gas exchange, and the oxygen carrying capacity of the blood, i.e. haemoglobin concentration.
Cardiac output increases linearly with VO2 in most patients, as does arterial mixed venous oxygen difference until a peak oxygen extraction ratio of 75% is reached. Therefore the slope of VO2 increase closely approximates to the patient’s ability to increase cardiac output to meet the requirements of increasing exercise. With increasing exercise, oxygen demand will begin to exceed supply, and ATP will be generated anaerobically. Theoretically the onset of anaerobic metabolism can be measured in three ways: an increase in blood lactate concentration, a decrease in arterial bicarbonate concentration as the lactic acid is buffered, and an increase in CO2 production as a result of the buffering of lactic acid by bicarbonate.
With rapid breath-to-breath analysers this increase in CO2 production can be identified without the requirement for direct measurement of blood levels of lactate and bicarbonate. The term ‘anaerobic threshold’ (AT) relates to a point during increasing exercise where the increase in lactate is accompanied by an almost equal reduction in the concentration of bicarbonate, and an increase in CO2 production i.e. CO2 production (VCO2) increases independently of VO2. This phenomenon was used by Beaver and colleagues to estimate the AT using the concept of the V slope [8], which is still used today.
How is the response to exercise relevant in CPET to the response to surgical stress? Major urological surgery promotes a profound physiological reaction mediated by an alteration in catabolic and anabolic hormone balance. The secretion of cortisol and catecholamines leads to an acute-phase response associated leading to a state of hypermetabolism [9]. Furthermore, global oxygen uptake can increase by up to 50% following major surgery [10]. Therefore if CPET demonstrates a patient has a poor aerobic capacity, in the form of a poor AT or peak VO2 (discussed below), then the ability of these patients to respond to this state of hyper-metabolism and increased oxygen demand is reduced, resulting in a higher likelihood in the complications seen following radical cystectomy, i.e. postoperative ileus, respiratory failure, cardiac failure and metabolic derangement.
Key variables from CPET for preoperative risk assessment are anaerobic threshold (AT) and peak VO2.
Anaerobic threshold ml.min-1kg-1
Anaerobic threshold is an objective measure of aerobic capacity, not influenced by effort. Several studies have demonstrated an AT of at least 11ml.kg.min is necessary to safely undertake significant surgery. Older and colleagues demonstrated that an AT<11ml.min-1kg-1 in elderly patients undergoing major intra-abdominal surgery was associated with a postoperative mortality of 42% in the presence of myocardial ischemia, and 18% without ischaemia [11]. In radical cystectomy Prentis et al. have demonstrated that an AT of 12ml.min-1kg-1 was the optimal predictor of major postoperative complications in their cohort of 82 patients [12].
It is therefore clear that not only is AT useful in helping to identify high-risk patients so we can give them a better indication of their personal risk associated with their surgery, it also helps us to identify patients who need critical care support after surgery. Figure 2 demonstrates how AT and peak VO2 could be used for triaging patients.
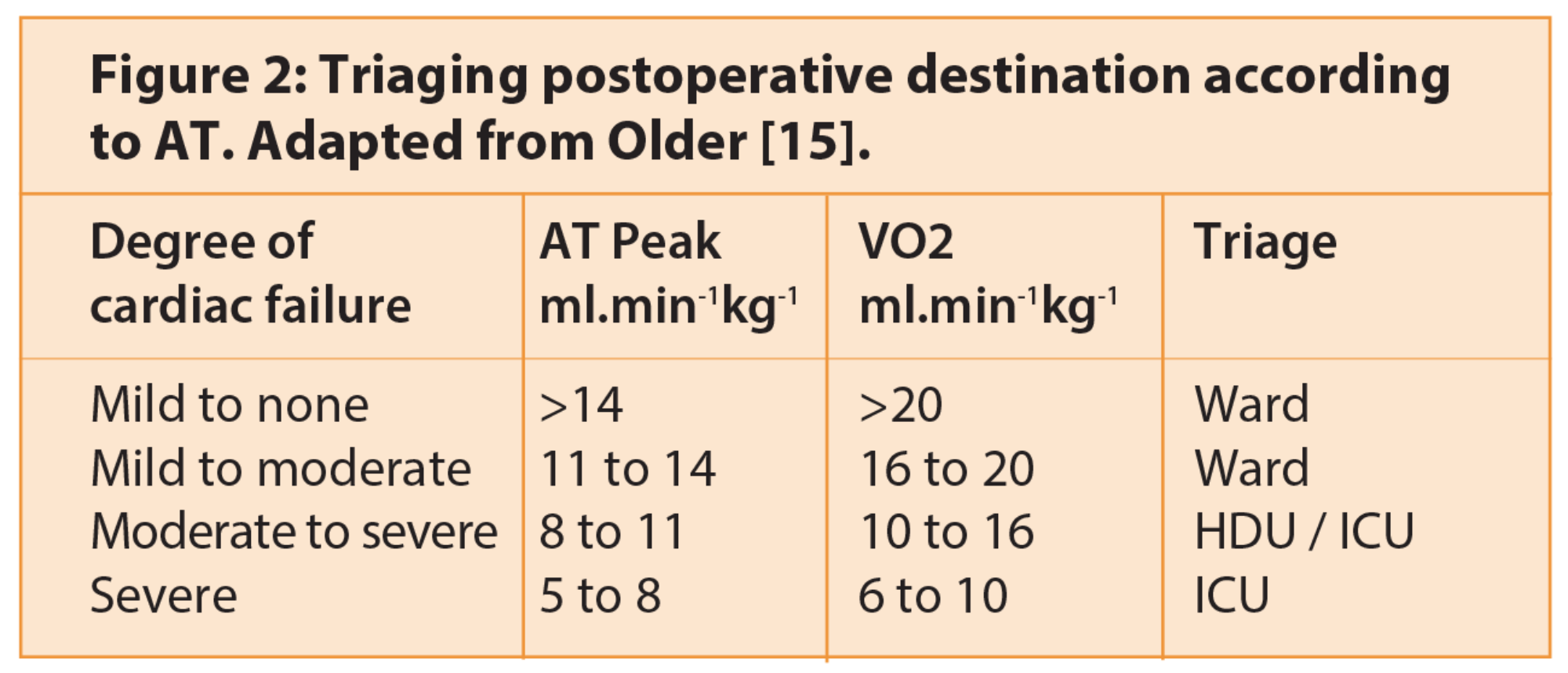
By targeting this high-risk group with high dependency unit (HDU) care, this will not only improve patient outcome but also potentially reduce financial cost, reducing complication rate and reducing length of stay. Many studies have shown that mortality following major surgery is related to the concept of ‘failure to rescue’. Complication rates between major teaching hospitals in America and small institutions were no different [13]; however, it was the failure to identify and promptly treat complications that was crucial to improving surgical outcome, and therefore morbidity. Having high-risk patients in a unit, such as critical care or a post-anaesthetic care unit (PACU), dedicated to the provision of high level care for post-surgical patients, with staff trained to focus on issues such as pain, fluid balance, and nutrition and respond appropriately to changes in physiological variables in the form of continuous blood pressure, arterial blood gas, SpO2 and ECG monitoring, therefore appears a crucial component of an ERAS protocol for major surgery aimed at improving surgical outcomes.
Peak VO2
Peak VO2 has been used predominantly as a measurement in relation to heart failure and cardiac transplant patients. Heart failure patients with a VO2 max <14.5ml/min/kg have a mortality rate double that of patients with a VO2 max >14.5ml/min/kg [14]. The issues with peak VO2 are that Older and colleagues have found that meaningful values in the elderly are hard to obtain, and that it is limited by patient motivation and symptoms limiting the CPET process.
Who should we test? In a recent review Older suggests we should test everybody over the age of 60 undergoing major surgery [15]. However, a national survey of practice in England undertaken in 2011 and published last year, suggests only 32% of all adult anaesthetic departments have access to CPET testing and 4% were in the process of setting CPET up [16]. This therefore underlines that although CPET is useful in risk stratification, it is by no means replacing clinical examination or clinical judgement. Furthermore Older raises a word of caution in viewing the AT as a binary triage tool, and suggests that too much emphasis must not be placed on an AT threshold of 11 as an indicator that a patient is fit to be managed on a ward [15].
Preoperative carbohydrate loading and fasting
While there is no study evaluating carbohydrate loading in cystectomy patients, it has been shown that such preoperative loading decreases thirst, reduces insulin resistance and helps maintain lean body mass and muscle strength in colorectal surgery. It involves the use of clear carbohydrate drinks the day prior to surgery and up to two hours before. In addition to the metabolic effects, it facilitates accelerated recovery through early return of bowel function and shorter hospital stay [17]. The carbohydrate drinks are specially formulated for rapid gastric transit with low osmolality. The practice of routinely fasting patients from midnight due to concern of airway soiling from aspiration is now out-dated. Current consensus from the Association of Anaesthetists of Great Britain and Ireland (AAGBI) is that two hours fasting for liquids and six hours for solids is correct practice [18].
Preoperative oral mechanical bowel preparation
There is no evidence supporting the routine use of bowel preparation in radical cystectomy patients. There was no difference between morbidity or length of stay in a prospective study comparing 30 patients who had undergone standard three-day bowel preparation and 32 patients who received no bowel preparation [19].
Intraoperative care
Goal-directed fluid therapy (GDFT)
GDFT using cardiac output monitors, such as a transoesophageal Doppler device (TODP) or LiDCO systems to guide fluid and inotropic therapy, has been shown to improve outcomes and reduce complication rates and length of hospital stay [20]. Used in conjunction with invasive arterial pressure monitoring, and central mixed venous oxygen saturations from a central venous pressure line, intraoperative individualised fluid therapy aims to optimise cardiac output, and therefore tissue perfusion and oxygenation.
Many centres have fluid challenge protocols to achieve supra-normal cardiovascular function, whereby the TODP is used to establish baseline values for stroke volume and FTC, among other parameters, and then a 250ml fluid bolus is given rapidly through either a large bore cannula or central venous line. If this results in an increase of 10% or more in the initial stroke volume, then a further fluid challenge can be given until there is no longer an increase of 10% or more in stroke volume. This optimisation in stroke volume and therefore cardiac output focuses on the concept of blood flow being the integral determinant of tissue perfusion rather than arterial blood pressure in isolation. By optimising blood flow to tissues, GDFT aims to improve gut perfusion, thereby reducing the incidence of hypo-perfusion and therefore occult bowel ischaemia and postoperative ileus, and allows the anaesthetists a better guide as to how the patient is responding to the significant fluid shifts that occur during radical cystectomy.
This fluid optimisation is thought to reduce the development of multi-organ dysfunction postoperatively and therefore reduce morbidity and mortality. In fact the National Institute for Health and Care Excellence (NICE) has issued guidelines recommending the use of the Cardio Q-ODM (Deltex Medical) oesophageal Doppler device for use in patients undergoing major or high risk surgery [21]. Furthermore in 2012, the Enhanced Recovery Partnership issued perioperative fluid guidance supporting the use of individualised GDFT as an integral part of an ERAS protocol, citing that using such technologies ensures that not only do we avoid hypovolaemia but also fluid excess, which itself is also harmful as it causes pulmonary oedema and increasing interstitial gut wall oedema which can lead to nausea and delay in return of gut motility [22].
Analgesia
No prospective single-intervention study has been conducted to assess epidural analgesia in the perioperative management of radical cystectomy, however there has been strong evidence shown in open colorectal surgery that epidural analgesia reduces the stress response to surgery, provides superior pain relief, reduces postoperative complications and accelerates functional recovery [23]. Recent ERAS society recommendations strongly encourage the use of T9-T10 thoracic epidural for radical cystectomy for 72 hours after surgery, as the benefits listed are key components in delivering an effective ERAS protocol. Epidural analgesia in combination with paracetamol and non-steroidal anti-inflammatories (where there are no contraindications) reduces, and often removes the need for systemic opioid analgesia, and its associated side-effects of bowel dysfunction, respiratory depression and nausea. More recently alternative strategies, such as intra-thecal analgesia, transversus abdominal plane blocks and continuous infusion of local anaesthetic via wound catheters have been proposed as alternatives to epidural analgesia for minimally invasive surgery, but there is limited evidence for their comparative efficacy to epidural analgesia in the context of an ERAS protocol.
Postoperative care
As discussed earlier, caring for the needs of the high-risk surgical patient are likely to be best met in a critical care or PACU environment. The staffing levels and facilities available make the delivery of a structured approach to the immediate postoperative care of these patients focusing on pain relief, bowel care and goal directed fluid therapy as part of an ERAS protocol more attainable than in a ward-based environment.
Avoidance of nasogastric tubes in major abdominal surgery has been shown to cause fewer postoperative complications and, where prophylactic NG tube had been maintained there was no advantage, according to a recent Cochrane meta-analysis [24]. Many centres now remove NG tubes at the end of surgery in radical cystectomy, to avoid delayed gastric emptying, nausea and vomiting that would otherwise delay patient mobilisation and therefore participation in an ERAS protocol.
Establishing normal food intake as soon as is possible after surgery is an essential component of enhanced recovery. There is no evidence that early enteral nutrition increases the incidence of anastomotic leak or dehiscence rate in colorectal surgery, in fact early enteral intake is thought to maintain the integrity of the gut mucosa, and therefore potentially reduce the likelihood of anastomotic break down. Routine prolonged fasting post-cystectomy is not recommended; early free fluids and diet as soon as tolerated should be encouraged [1]. Early mobilisation, enabled through the use of epidural analgesia as effective pain relief, should be encouraged as it is clear that prolonged bed rest increases the risk of postoperative thromboembolism in this high-risk group, and the risk of pulmonary complications.
Nurse specialists play a key role in engaging patient participation in their stoma care in the initial perioperative period. One centre reports that early visits, from day one, ensure patients feel supported in coming to terms with the appearance of their stoma, and from day two patients are encouraged to engage in their stoma care, for example changing the stoma pouch [9]. They report that by day five patients are ready and managing their stoma, thereby removing any issues surrounding ability to self-care that may delay discharge as part of an enhanced recovery protocol.
Conclusion
ERAS protocols have been adopted in many surgical specialties, particularly colorectal surgery, with improvements in mortality and morbidity. This article highlights that a multi-modal, structured approach to perioperative care of the patient undergoing robotic radical cystectomy is likely to improve morbidity and mortality, and shorten hospital stay. Furthermore identifying high-risk patients through CPET for whom a higher level of postoperative care will be of benefit, is likely to focus scarce critical care resources on patients for whom ward-based care may not be suitable. Implementing an ERAS protocol that reduces postoperative morbidity and consequently reduces length of hospital stay, will lead to patients leaving hospital faster, and fitter following surgery. Additionally it will deliver an improved patient experience and result in financial savings and service capacity benefits.
References
1. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS®) society recommendations. Clinical Nutrition 2013;32(6):879-87.
2. Sahabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for pateints with bladder cancer using a standardized reporting methodology. European Urology 2009;55(1):164-74.
3. Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer long term results in 1,054 patients. Journal of Clinical Oncology 2001;19(3):666-75.
4. Menon M, Hemal AK, Tewari A, et al. Nerve sparing robotic robot-assisted radical cystoprosatectomynad urinary diversion. BJU International 2003;92(3):232-6.
5. Li K, Lin T, Fan X, et al. Systematic review and meta-analysis or comparative studies reporting early outcomes after robot assisted radical cystectomy versus open radical cystectomy. Cancer Treatment Reviews 2012;39(6):551-60.
6. Belardinelli R, Lacalpricea F, Carleb F, et al. Exercise induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J 2003;24:1304-13.
7. Wilson RJ, Davies S, Yates D, et al. Impaired functional capacity is associated with all cause mortality after major elective abdominal surgery. Br J Anaesth 2010;105(3):297-303.
8. Beaver WL, Wasserman K, Whipp BJ. Online computer analysis and breath by breath graphical display of exercise function tests. J Appl Phys 1973;34(1):128-32.
9. Vasdev N, Pillai P, Snowdon CP, Thorpe AC. Current Strategies to Enhance Recovery following Radical Cystectomy: Single Centre Initial Experience. ISRN Urology 2012;1-6.
10. Older P, Smith R. Experience with the pre-operative invasive measurement of haemodynamic, respiratory and renal function in 100 elderly patients schedule for major abdominal surgery. Anaesth Intensive Care 1988;16(4):389-95.
11. Older P, et al. Pre-operative evaluation of cardiac failure and ischaemic in elderly patients by CPET. Chest 1993;104:701-4.
12. Prentis JM, Trenell MI, Vasdev N, et al. Impaired cardiopulmonary reserve in an elderly population isrelated to postoperative morbidity and length of hospital stay after radicalcystectomy. BJUI 2013;112:E13-19.
13. Ghaferi AA, Birkmeye JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. New Engl J Med 2009;361(14):1368-75.
14. Burnside, Snowden. Physiological basis of preoperative cardiopulmonary exercise testing. Surgery 2014;32:59-62.
15. Older P. Anaerobic Threshold, is it a magic number to determine fitness for surgery? Peri-operative Medicine 2013;2:2-13.
16. Huddart S, Young EL, Smith RL, et al. Preoperative cardiopulmonary exercise testing in England - a national survey. Perioper Med (Lond) 2013;2(1).
17. Nygren J, Thorell A, Ljungqvist O. Preoperative oral carbohydrate nutrition: an update. Curr Opin Clin Nutr Metab Care 2001;4:255-9.
18. AAGBI Guideline: Pre-operative Assessment and Patient Preparation – The role of the Anaesthetist 2. 2010.
19. Tabibi A, Simforoosh N, Bashiri A, et al. Bowel Preparation verus no bowel preparation before ileal urinary diversion. Urology 2007;70(4):654-8.
20. Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid managements shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005;95(5):634-42.
21. NICE. NICE medical technology guidance 3:CardioQ-ODM oesophageal doppler monitor. 2011.
22. Mythen MG, Swart M, Acheson N, et al. Peri-operative Fluid Management: Consensus Statement from the enhanced recovery partnership. Perioperative Medicine 2012;1:2-4.
23. Carli F, Kehlet H, Baldinin G, et al. Evidence basis for regional anaesthesia in multi-disciplinary fast-track surgical pathways. Regional Anaesthesia and Pain Medicine 2010;36(1);63-72.
24. Nelson R, Edwards S, Tse B. Prophylactic nasogastric decompression after abdominal surgery. Cochrane Database of Systematic Reviews 2007;3:CD004929.
Declaration of competing interests: None declared.






