
Prostate cancer (PCa) is the second most common cancer in the UK, with 43,000 cases in 2017-18 [1,2]. Accurate primary staging and the detection of suspected recurrence following treatment is vital for directing management and predicting prognosis.
This has conventionally been dependent upon digital rectal examination (DRE), prostate specific antigen (PSA) testing and prostate biopsy, either by the transrectal, or increasingly, the transperineal route. This pathway is now complemented with imaging, including multiparametric MRI (mpMRI), computed tomography (CT) and bone scintigraphy (BS).
Despite the excellence of mpMRI for local staging, anatomical imaging predominantly depends on morphological features, and lesions smaller than 8-10mm (such as lymph nodes) are frequently missed [3]. Malignant bone lesions may have little uptake on imaging and benign changes can be mistaken for metastases.
Over the last 20 years, there has been an increased interest in positron emission tomography (PET). Whist not new (TIME Magazine named PET-CT the medical invention of the year in 2000), recent developments in radiopharmacy have positioned it at the forefront of cancer diagnostics and therapy. In this article, PET radiopharmaceuticals and their use in the primary staging and biochemical recurrence of prostate cancer will be explored.
PET basics
PET is a nuclear medicine technique that measures physiological function, by evaluating parameters such as blood flow, cellular metabolism and neurotransmitter activity.
Radioactivity is detected after a substance named a ‘tracer’ is radio-labelled and delivered to the body (typically intravenously). Tracers emit a signal and may be taken up by tissues due to metabolic changes induced by a disease process. PET camera systems contain a ring of detectors that encircle the patient and data from these signals are reconstructed into an image. Radiotracers are produced in either a particle accelerator (cyclotron) or a nuclear-reactor (generator). The radioactive dose is similar to that of CT (8mSV).
The early days of PET imaging were plagued by the difficulty in accurately localising the precise origin of radiotracer uptake. Anatomical fusion was therefore developed alongside PET and in 1991, David Townsend and colleagues at the University of Geneva conceived the first hybrid PET-CT scanner. Today, almost invariably, an accompanying attenuation correction CT scan is obtained with the PET.
PET radiopharmaceuticals
Fluorodeoxyglucose (18F-FDG)
18F-FDG is a radiolabelled analogue of glucose, that is trapped within cells in proportion to the rate of aerobic glycolysis. Cancer cells typically utilise this metabolic pathway to a greater extent than non-cancerous cells (the Warburg effect) and the tracer therefore preferentially localises to malignant tissue.
There is a long-held consensus that FDG PET-CT is not useful in prostate cancer, as initial studies reported poorer results compared to those from other neoplasms. However, further experience has demonstrated that FDG PET-CT may be useful in specific phases of the disease and there is a renewed interest in its use.
Radiolabelled choline analogues (11C /18F-Choline)
Choline is a water-soluble nutrient, which enters cells via cell surface transporters, and is phosphorylated by choline kinase to phosphocholine – the major phospholipid component of cell membranes. There is an increased uptake of choline in prostate cancer cells.
The major advantages of 11C-Choline are its minimal urinary excretion, which enables a more accurate assessment of the prostate bed and pelvic nodes. However, it has a short half-life of only 20 minutes, which limits use to institutions with cyclotrons.
18F-Fluciclovine
18F-Fluciclovine (18F-FACBC) is a radiolabelled synthetic amino acid analogue, initially developed to image brain tumours, whose utility in prostate cancer was discovered incidentally during a study of renal cancer. 18F-Fluciclovine uptake reflects increased expression of amino acid transporters on prostate cancer cell membranes, with greater expression in tumours with a higher Gleason score.
18F-Fluciclovine has a low urinary excretion and minimal bladder activity, improving its ability to detect local disease.
Prostate specific membrane antigen
Prostate specific membrane antigen (PSMA) is a transmembrane glycoprotein. Dysplastic or neoplastic transformation of prostate tissue results in the transfer of PSMA from the apical membrane to the luminal surface of the prostatic duct. Whilst PSMA is expressed on prostate epithelium in 80-90% of adenocarcinomas, the name is a slight misnomer. It is also present on the vascular endothelial cells of both normal tissue and tumours including the renal and transitional cell and on astrocytes in the central nervous system.
PSMA expression is greatest and most homogeneous in high-grade tumours and expression can be confirmed with immunohistochemistry.
68Ga-labelled PSMA radiopharmaceuticals
68Ga-PSMA-11 was first described in 2012 and is the most studied and widely used PSMA PET radiopharmaceutical. 68Ga-PSMA-11 is excreted by the kidneys and has intense activity in the urinary tract. Ureteric activity can be mistaken for pathological nodal uptake, whilst bladder activity can make prostate bed assessment difficult.
Imaging is typically performed at 60 minutes, although delayed imaging increases uptake and contrast, which could be useful for delineating equivocal lesions.
18F-labelled PSMA radiopharmaceuticals
Despite the success of 68Ga-labelled PSMA radiopharmaceuticals, they are not without their disadvantages. A unit must consider the cost of a generator and the relatively short half-life of gallium (68 minutes), which limit the number of doses that can be produced per day. The development of cyclotron-produced 18F-labelled PSMA, can allow a greater number of examinations, due to higher amounts of available radioactivity from a cyclotron, the longer half-life of 18F and improved spatial resolution and image quality.
Staging of primary prostate cancer
Multiparametric MRI is the imaging modality of choice for patients with suspected clinically localised CaP. Assessment of extra-prostatic disease is vital prior to embarking upon curative treatment and a number of current guidelines recommend CT and BS in patients with intermediate and high-risk CaP [4]. PET-CT, however, is challenging this status quo.
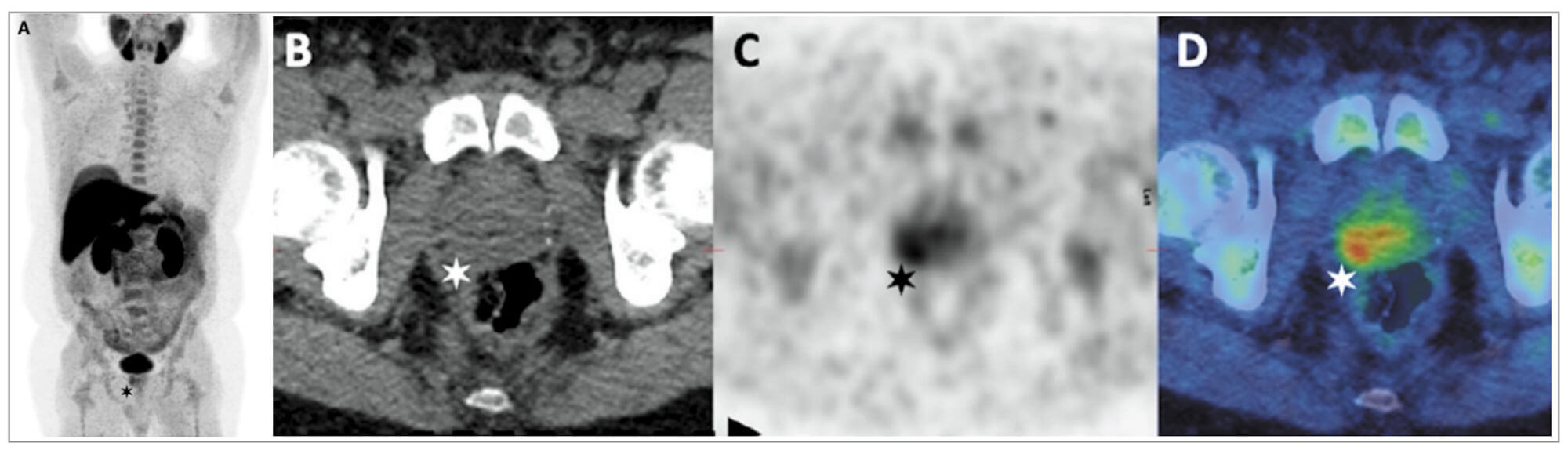
Figure 1: Choline PET-CT in staging of CaP. PSA 9.3ng/mL, T2aN0 on mpMRI, GS 4 + 3.
A & C: PET B: Axall CT D: Fused PET-CT images
Images demonstrate a choline-avid right basal prostatic tumour.
Radiolabelled choline analogues (11C /18F-Choline)
Choline PET-CT is superior to conventional imaging for extra-prostatic disease but has limited accuracy in differentiating between benign and malignant intra-prostatic pathologies (Figure 1). Prospective studies in patients with intermediate and high-risk CaP, have reported reasonable sensitivities (45-73.2%) but high specificities (87.6-96%) for nodal disease [5]. A meta-analysis of 10 choline PET-CT studies, in a total of 441 patients, confirmed a pooled sensitivity of 49% but a high specificity of 95% for nodal disease [6].
18F-Fluciclovine
Small, prospective, single centre studies have confirmed higher uptake in tumour foci compared to normal tissue, but a similar uptake in benign prostatic hyperplasia, thereby limiting specificity. A multicentre trial of 68 patients with CaP (42 patients awaiting radical prostatectomy – RP) reported that none of the seven RP patients with histologically-proven nodal disease demonstrated 18F-Fluciclovine uptake in the largest node. This reflects an inability to give a signal where tumour burden is low and demonstrates the limitations of the spatial resolution of PET with this tracer. A meta-analysis confirmed a pooled sensitivity of 87% and specificity of 84% for primary tumour detection, and pooled sensitivity of 56% and specificity of 98% for nodal staging [7].
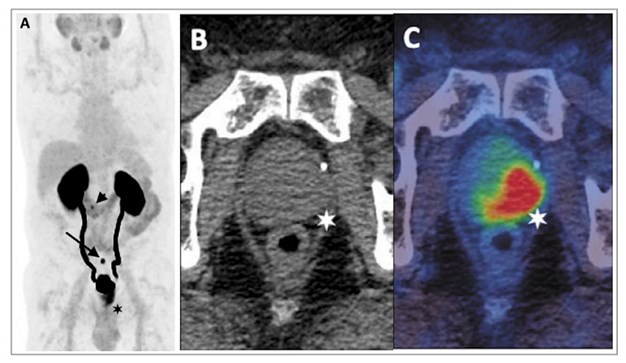
Figure 2: 68Ga-THP-PSMA PET-CT in staging of primary prostate cancer.
A: PET image B: Axial CT C: Fused PET-CT image
Images demonstrate high-grade uptake in a predominantly left-sided prostatic tumour
with low-grade uptake in a 6mm left internal iliac node (arrow on PET, star on fused images).
68Ga-labelled PSMA radiopharmaceuticals
There are an increasing number of studies demonstrating the superiority of 68Ga- labelled PSMA PET-CT over conventional imaging for CaP staging (Figure 2). A prospective study of 113 patients confirmed significantly higher sensitivity (96.2% vs. 73.1%) and accuracy (99.1 vs. 84.1%) using 68Ga-labelled PSMA PET-CT compared with BS for metastatic bone disease [8].
Several studies have assessed the diagnostic performance of 68Ga-labelled PSMA PET-CT for nodal staging with histopathological validation. The largest series of 208 patients reported a sensitivity for nodal disease of 38.2% (21 of 55 patients) and a per node sensitivity of 24.4% (42 of 172 nodes). The median diameter of a malignant node was 4.8mm, with 15% of histologically-proven malignant nodes measuring <5mm [9].
The ProPSMA study, an Australian, multicentre, prospective, randomised controlled trial, of 300 patients with a primary CaP, compared 68Ga-labelled PSMA PET-CT with conventional imaging [10]. PSMA PET-CT had a 27% greater accuracy compared to conventional imaging (92% vs. 65%). The results of conventional imaging resulted in a treatment change for 23 men compared with 41 men following PSMA PET-CT. Conventional imaging was also associated with a higher radiation dose (19.2mSv compared to 8.4mSv). The group have also reported data on favourable cost outcomes of PSMA PET-CT, further demonstrating the clinical utility of this technique [10].
68Ga-labelled PSMA PET-CT has been shown to have a significant impact on management, with Roach et al. demonstrating a 21% change in management intent and Kulkarni et al. a 24% change respectively [11-12].
Biochemical recurrence
Following either RP or radiotherapy (RT), between 27-53% of patients develop a biochemical recurrence (BCR). Whilst a rising PSA level typically precedes metastatic progression, PSA-only recurrence may be prolonged and a measurable PSA may not necessarily lead to identifiable metastatic disease.
The PSA level that defines treatment ‘failure’ depends upon the primary intervention, with a rising level after RP or primary RT, requiring different approaches in investigation and management.
After RP, the threshold that best predicts further metastases is a PSA >0.4ng/mL, which continues to rise. Patterns of PSA recurrence, such as a short time interval from prostatectomy (<3 years), high PSA velocity, and a short doubling time (<3 months), suggest the presence of metastatic disease. After primary RT, with or without short-term hormonal manipulation, the RTOG-ASTRO Phoenix Consensus Conference definition of PSA failure (with an accuracy of >80% for clinical failure) is any PSA increase >2ng/mL higher than the PSA nadir value. Imaging is only of value if it leads to a treatment change which results in an improved outcome for the patient. The ability to detect recurrent disease at low PSA levels has been one of the major successes of PET-CT (Figure 3).
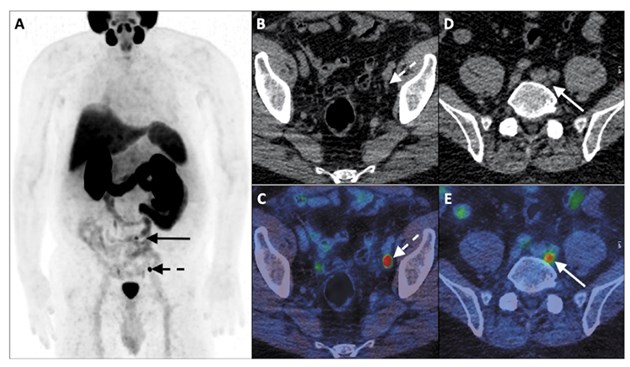
Figure 3: 68Ga-PSMA-11 PET-CT in the biochemical recurrence setting. GS 3 + 4 CaP,
post-RP (pT2N0M0) with PSA rise to 3.7ng/mL.
A: PET B & D: Axial CT C & E: Fused PET-CT
Images demonstrate an intensely PSMA avid 6mm external iliac node and
moderately PSMA avid 6mm left common iliac node.
Standard imaging with bone scan and MRI has a low pick-up rate in men with a PSA below 2ng/mL. However, PSMA PET/CT has been shown to identify residual cancer with positivity rates of 33%, 46%, 57%, 82%, and 97%, in men with post-RP PSA ranges of 0-0.19, 0.2-0.49, 0.5-0.99, 1-1.99, and >2ng/mL, respectively.
11C/18F-Choline PET-CT has demonstrated its superiority over conventional imaging in BCR, with several meta-analyses confirming high diagnostic accuracy. A meta-analysis by Fanti et al. reported an overall detection rate of 62%, with a pooled sensitivity and specificity of 89% [13]. Changes in management have also been demonstrated. A prospective multicentre trial assessing the impact of Choline PET-CT in 179 patients with BCR, with negative or equivocal conventional imaging, reported a 56% change in treatment. A 150-patient retrospective study assessing the impact of 11C-Choline PET-CT in BCR, reported a slightly lower overall clinical impact of 46.7% (70/150 patients), with 38.6% (27/70) of decisions classed as a major management change (switch from salvage to palliative therapy or vice versa) and 61.4% (43/70) as minor [14].
68Ga-PSMA PET-CT has also demonstrated its superiority to conventional imaging and indeed other PET radiopharmaceuticals in BCR, particularly at low PSA levels. Detection rates of between 11.3-58.3% at PSA levels <0.ng/ml and 11.0-65.0% at PSA levels <0.5ng/ml have been reported [15].
In a meta-analysis conducted by Pereira et al. pooled detection rates of 33% PSA <0.2ng/ml, 45% PSA 0.2-0.5ng/ml, 95% >2.0ng/ml were reported, with similar results by Hope et al, which included only studies with histological validation, demonstrating a pooled sensitivity of 99%, specificity of 76% and a 40% detection rate at a PSA level <0.2ng/ml [16,17].
Given the substantial morbidity of salvage treatments, distant metastases must be ruled out in patients with local recurrences and who are fit for these options. After RP, PSMA PET-CT appears to be the imaging modality with the highest sensitivity at low PSA levels (<0.5ng/mL) and may help distinguishing patients with recurrences confined to the prostatic fossa from those with distant metastases. After RT, MRI has shown excellent results at detecting local recurrences and guiding prostate biopsy. Choline, fluciclovine or PSMA-PET-CT can be used to detect metastases.
No hard and fast rules exist globally regarding the use of PET-CT imaging in the context of BCR, due to the relative novelty of this technique and lack of data from prospective studies.
Following RP, PSMA PET-CT can be performed if the PSA level is >0.2ng/mL and the results will influence subsequent treatment decisions. If this modality is unavailable, and the PSA level is >1ng/mL, fluciclovine or choline PET-CT can be done. Negative imaging however, should not delay treatment if otherwise indicated.
After RT, MRI has shown excellent results at detecting local recurrences and guiding prostate biopsy. Distant metastases must also be ruled out in patients with local recurrences and who are fit for these salvage therapies. Choline, fluciclovine- or PSMA-PET-CT can be used to detect metastases in these patients, and local expertise can guide this.
Clinical considerations of PET imaging
Despite the huge enthusiasm surrounding PET imaging, a greater evidence base is still required, validating imaging with a reference standard such as histopathology. The implications of identifying metastatic disease on primary and recurrent staging also require exploration. We must exercise caution when interpreting imaging findings and avoid repeating the mistake of overtreatment.
To widely implement novel technologies, several logistical barriers must be addressed, including issues around cost-effectiveness and equal access. Tracer development requires commercialisation, complex operational procedures, and scan performance and interpretation need to be standardised. PET imaging is not globally available, mainly owing to regulatory issues, which only allow its application under certain restrictions. Very few centres can therefore use these novel tracers outside of clinical trials. Thus, the design and conduction of prospective trials are a high priority, so that evidence required to enable implementation of this promising imaging technology into clinical guidelines is obtained.
Conclusion
PET-CT in prostate cancer has demonstrated its superiority to conventional imaging techniques in both the primary staging and BCR setting.
The modality offers high disease detection rates at low PSA levels, with a resultant impact on patient management. Novel PET radiopharmaceuticals continue to be developed and PSMA inhibitors are increasingly being used as ‘theranostic’ agents in patients with metastatic prostate cancer, to both visualise and treat lesions simultaneously.
Despite the growing body of evidence demonstrating the superiority of PSMA PET radiopharmaceuticals, they remain without full FDA, EMA or NICE approval. Though much has yet to be learned, PET imaging holds great promise in the diagnosis and treatment of prostate cancer.
TAKE HOME MESSAGE
-
PET imaging can add molecular information to the anatomical information from multiparametric MRI and therefore aid the delineation of suspicious lesions for biopsy.
-
PET-CT imaging shows increased specificity and sensitivity compared with current standard imaging (CT, MRI and bone scintigraphy) in patients with primary intermediate or high-risk prostate cancer.
-
PET-CT imaging improves the detection of metastatic lesions at low serum PSA values in biochemically recurrent prostate cancer.
-
Prostate-specific membrane antigen (PSMA) is emerging as the most promising and specific target for prostate cancer PET imaging.
References
1. National Prostate Cancer Audit Annual Report 2019.
https://www.npca.org.uk/
reports/npca-annual-report-2019/
[accessed 10 March 2020].
2. Cancer Research UK.
https://www.cancerresearchuk.org/
health-professional/cancer-statistics/
statistics-by-cancer-type/prostate- cancer
[accessed 10 March 2020].
3. Hovels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol 2008;63(4):387-95.
4. EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer.
https://uroweb.org/guideline/
prostate-cancer/
[accessed 15 March 2021.]
5. Beheshti M, Imamovic L, Broinger G, et al: 18F Choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: A prospective study of 130 patients. Radiology 2010;254:925-33.
6. Evangelista L, Guttilla A, Zattoni F, et al. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: A systematic literature review and meta-analysis. Eur Urol 2013;63:1040-8.
7. Kim SJ, Lee SW. The role of 18F-fluciclovine PET in the management of prostate cancer: A systematic review and meta-analysis. Clin Radiol 2019;74:886-92.
8. Lengana T, Lawal IO, Boshomane TG, et al. 68Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: A fait accompli? Clin Genitourin Cancer 2019;16:392-401.
9. Yaxley JW, Raveenthiran S, Nouhaud FX, et al. Outcomes of primary lymph node staging of intermediate and high risk prostate cancer with 68Ga-PSMA positron emission tomography/computerized tomography compared to histological correlation of pelvic lymph node pathology. J Urol 2019;201:815-20.
10. Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020;395(10231):1208-16.
11. Roach PJ, Francis R, Emmett L, et al. The Impact of (68)Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med 2018;59:82-8.
12. Kulkarni M, Hughes S, Mallia A, et al. The management impact of 68gallium-tris(hydroxypyridinone) prostate-specific membrane antigen (68Ga-THP-PSMA) PET-CT imaging for high-risk and biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2020;47(3):674-86.
13. Fanti S, Minozzi S, Castellucci P, et al. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: Meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging 206;43:55-69.
14. Gillebert Q, Huchet V, Rousseau C, et al. 18F-fluorocholine PET/CT in patients with occult biochemical recurrence of prostate cancer: Detec- tion rate, impact on management and adequacy of impact. A prospective multicentre study. PLoS One 2018;13:e0191487.
15. De Visschere PJL, Standaert C, Futterer JJ, et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol 2019;2:47-76.
16. Pereira Mestre R, Treglia G, Ferrari M, et al. Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: A meta-analysis. Eur J Clin Invest 2019;49:e13063.
17. Hope TA, Goodman JZ, Allen IE, et al. Metaanalysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med 2019;60:786-93.
Declaration of competing interests:
The authors acknowledge financial support for research work, from the King’s College London / University College London Comprehensive Cancer Imaging Centres funded by Cancer Research UK and Engineering and Physical Sciences Research Council in association with the Medical Research Council and the Department of Health and the Wellcome Trust EPSRC Centre for Medical Engineering at King’s College London. Meghana Kulkarni is part funded by Theragnostics.





