Life expectancy in men diagnosed with de novo synchronous metastatic hormone-sensitive prostate cancer (mHSPC) has risen to a median of 4.8 years with upfront systemic agents (such as docetaxel) in addition to standard androgen deprivation therapy (ADT) [1-3]. Within this context, the uro-oncology research community has moved to explore the role of local cytoreductive surgical treatments, with or without, metastasis-directed therapy (surgical metastasectomy or stereotactic ablative body radiotherapy [SABR]).
These additional cytoreductive treatments are hypothesised, but not proven, to provide added oncological benefit to existing systemic therapy, which over time may lead to the development of a castrate-resistant state [4-8].
In particular, a subgroup of men with de novo synchronous ‘oligometastatic’ or ‘low volume’ metastatic disease may achieve localised and potentially distant cancer control following the use of various cytoreductive treatment strategies [9,10]. The oligometastatic state is an arbitrarily defined state that arises between ‘locally-advanced’ and ‘poly-metastatic’ disease, attempting to demarcate the burden of metastases [9]. However, the definition lacks international consensus and is controversial, particularly given that a particular patient’s risk does not jump as a result of having a single extra metastasis [9]. Current definitions combine absolute number (e.g. ≥4 or ≤5), anatomical sites (e.g. nodal, bone, visceral), spatial patterns (e.g. axial vs. non-axial skeleton) and identification on diagnostic imaging (e.g. conventional vs. molecular targeted) [9,11].
At present, cytoreductive external-beam radiotherapy (EBRT) has the highest level of evidence to support its application [12]. The Systemic Therapy in Advancing Or Metastatic Prostate Cancer: Evaluation Of Drug Efficacy (STAMPEDE) collaborators explored the role of cytoreductive EBRT in 2061 patients with newly diagnosed metastatic prostate cancer (Arm-H). In this trial, all patients received ADT, with a minority (18%) also receiving upfront docetaxel [12]. In the low burden disease pre-specified subgroup of patients (CHAARTED definition), a significant overall survival (OS) advantage was achieved with cytoreductive EBRT compared to systemic agents alone (three-year OS 81% vs. 73%, HR 0.68, [95% CI 0.52–0.90]; p=0.007) [1,12]. Many have questioned the use of a subgroup, which would normally be hypothesis generating, to change clinical practice.
However, the chapter for cytoreductive surgery within this pathway is awaiting its conclusion with large randomised controlled trials (RCTs) incorporating cytoreductive radical prostatectomy (cRP) yet to report outcomes.
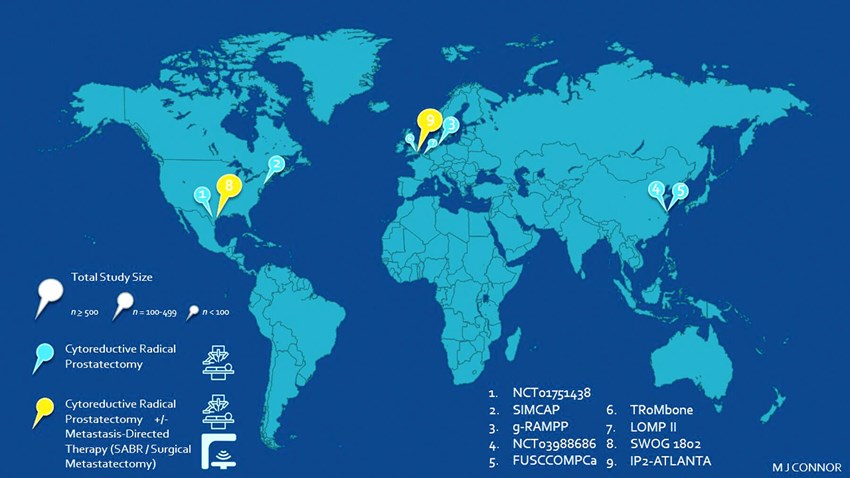
Figure 1: Global portfolio of randomised trials offering cytoreductive surgery
in de novo synchronous metastatic prostate cancer.
In this article, we describe the current global portfolio of cytoreductive surgery RCTs on offer for patients with de novo synchronous mHSPC (Figure 1). We discuss important differences in approaches, including use of systemic agents, timing of surgical intervention, use of extended pelvic lymph node dissection (ePLND), eligibility criteria, diagnostic imaging and finally multi-modal approaches integrating metastasis-directed therapy.
Early prospective non-comparator studies
To provide grounding, there have been numerous retrospective datasets and a single case-control study suggesting that cRP +/- ePLND has oncological promise, benefits in terms of locoregional symptom control (i.e. haematuria, bladder outflow obstruction, ureteric obstruction) and has an acceptable safety profile in a select sub-group of men with oligometastatic disease [5,13-17].
Furthermore, the Local Treatment of Metastatic Prostate Cancer (LoMP I) trial reported early feasibility in 21 patients, in whom ADT was commenced postoperatively at symptomatic or biochemical progression (NCT02138721) [18]. cRP + ePLND was accepted in 17/21 (81%). Operating on this cohort of exclusively ≤ T3b disease, in which the median bone metastases per patient were two (IQR 1-9), there were no intraoperative complications. Unsurprisingly, 14/17 (82.4%) of men had positive surgical margins, with pN1 present in 10/12 (70.6%) with a median of 10 (IQR 1-39) invaded nodes. Continence and the absence of locoregional symptoms was achieved in 12/17 (71%). Of note, no Clavien-Dindo grade >/= III complications were reported.
An overview of trial design
Overall, we identified nine randomised studies that can be broadly categorised into i) feasibility ii) local cytoreduction and iii) multi-modal treatment RCTs.
i) Feasibility RCTs
With respect to feasibility RCTs, the Testing Radical prostatectomy in men with prostate cancer and oligoMetastases to the bone (TroMbone; ISRCTN15704862; Oxford University, UK) [16] randomised 50 patients with one to three bone metastasis to standard systemic therapy (SST) + cRP + ePLND or SST alone. The primary outcome was feasibility and safety, with the study having completed recruitment.
Second, the cytoreductive prostatectomy versus cytoreductive prostate irradiation as a Local Treatment Option for Metastatic Prostate cancer (LoMP II; NCT03655886; University of Ghent, Belgium) is randomising 86 men to SST with either cRP or EBRT. The feasibility of randomisation is the primary outcome with study recruitment ongoing.
ii) Local cytoreduction RCTs
Beyond the feasibility RCTs, are those powered to report on either failure free-survival (FFS), progression-free survival (PFS), cancer-specific survival (CSS), or OS. Of note, there is significant heterogeneity in the composite definition of these respective endpoints, although some trials do mirror established definitions used in prior major oncology trials [12,19].
In this space, four trials are active, the phase II/III Surgery in Metastatic Carcinoma of Prostate study (SIMCAP; NCT03456843; New Jersey, USA) RCT is randomising 190 men with cT1-T3, M1a/b disease to SST + cRP +/- ePLND or SST alone, with FFS the primary endpoint. The study is designed as such that if the FFS demonstrates >/= 30% advantage, the phase III study will be triggered with OS as an endpoint.
In a similar fashion, the phase II Impact of Radical Prostatectomy as Primary Treatment in Patients With Prostate Cancer With Limited Bone Metastases (g-RAMPP; NCT02454543; Hamburg, Germany) was designed to randomise 452 men with bone metastases (1 to 5) to SST + cRP + ePLND or SST alone. Following the results of STAMPEDE Arm-H, this study was stopped early in accrual, with follow-up results from 131 who were recruited not yet published. Furthermore, the Testing Radical Prostatectomy in Chinese Men With Prostate Cancer and oligoMetastases to the Bone (NCT03988686; Fudan University, China) is allocating 120 men with 1-3 bone metastasis with cT1-3 disease to SST or SST + cRP. Primary outcome in this study is time to castrate resistant disease.
Two studies offer EBRT as an alternative in the experimental arm. First, the ADT or ADT Plus Definitive Treatment (FUSCC-OMPCa; NCT02742675; Fudan University; China) phase II study has completed randomisation of 200 patients with bone or nodal metastasis (1 to 5) to ADT only or ADT + Radical Therapy (cRP or ERBT). This trial will report on radiological-PFS and OS as a primary and secondary outcome, respectively. Second, the Best Systemic Therapy or Best Systemic Therapy (BST) Plus Definitive Treatment phase II trial from MD Anderson (NCT01751438; University of Texas; USA) has completed a recruitment of 180 men with all burden metastatic disease to SST or SST + radical therapy (cRP or EBRT). The primary outcome is PFS and results are awaited once the data is matured. The study has now moved onto become the SWOG 1802 RCT described below.
iii) Multi-modal treatment RCTs
Multi-modal treatment trials combine SST, local cytoreductive surgery and MDT (surgical metastasectomy or SABR). First, the phase III, Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer (SWOG 1802; Southwest Oncology Group; Texas; USA) is designed for 1273 patients with all burden disease to be allocated to either SST or SST + radical therapy (cRP or EBRT); patients may also undergo MDT to less than or equal to four metastatic sites prior to randomisation. This study is recruiting and will report on OS as a primary endpoint.
Second, the Additional Treatments to the Local tumour for metastatic prostate cancer: Assessment of Novel Treatment Algorithms (IP2-ATLANTA; NCT03763253; Imperial College London; UK) is a phase II, three-arm RCT of 918 patients [19]. This study incorporates an embedded feasibility pilot that has been completed (n=80), and of which results are awaited [20]. This trial has three arms: i) control (standard of care), ii) SST + minimally invasive ablative therapy (HIFU or cryotherapy) +/- ePLND +/- MDT and iii) radical therapy (cRP +/- ePLND or EBRT +/- pelvic lymph node radiotherapy [PLNRT]) +/- MDT. MDT is restricted to the pre-specified cohort of patients with low metastatic burden. SST is available upfront in all arms. Primary outcome is a composite definition of PFS. The embedded feasibility phase evaluated feasibility of randomisation, safety and proportion of patients with a negative biopsy and non-suspicious prostate MRI post-SST.
Diagnostic imaging
Local staging
Two studies have mandatory requirements for a prostate MRI. The TRoMbone study mandates prostate MRI in the experimental treatment arm whilst the IP2-ATLANTA study requires all patients to have a prostate MRI at diagnosis [16,19]. In IP2-ATLANTA the embedded feasibility cohort, all patients in the experimental treatment arms underwent a second prostate MRI post-SST. This post-SST prostate MRI was converted to optional in the main phase of the study. Both these studies will report on the radiological changes observed in the local tumour and pelvis following contemporary SST. In the case of IP2-ATLANTA this will be reported with correlation to repeat histological evaluation of the prostate. TRoMbone and IP2-ATLANTA will also provide data on the role of serial prostate MRI in planning for cytoreductive surgery [21].
Distant staging and metastatic burden
Distant disease staging is a rapidly evolving space with conventional imaging (such as CT and bone scintigraphy) being augmented by prostate-specific membrane antigen (PSMA)-targeted imaging in some academic centres [22]. This is particularly apparent in high-risk, presumed localised, prostate cancer following the results of the proPSMA study demonstrating superior diagnostic accuracy of first-line Ga68 PSMA-PET/CT over conventional imaging [22].
PSMA PET/CT imaging can identify ‘micro-‘ or ‘early-‘ oligometastatic disease, in the same vein ‘oligometastatic’ may also appear as ‘poly-metastatic’ [23]. This has a downstream impact on the accurate assignment of metastatic burden definitions, used in prior oncology studies. Therefore, generating robust distant staging criteria for a trial that also takes account of the changing landscape and potential risk of a ‘Will Rogers’ effect of stage specific migration is a significant challenge to clinical trialists [11,23].
SWOG 1802, NCT03988686, NCT01751438 and FUSCC-OMPCa are limited to disease identified on conventional imaging and MRI. The g-RAMPP and TRoMbone protocols allow for both conventional imaging and PET/CT. IP2-ATLANTA permits both modalities, but derives metastatic burden from conventional components of the imaging performed to mitigate against this stage migration effect. Similar to prior oncology trials, this trial has a pre-specified low-burden subgroup (CHAARTED definition) that will ensure equal low and high disease burden representation across all trial arms through stratified randomisation [1].
Standard of care comparators
Recent advances in systemic agents have led to a wide landscape of drug therapies which include new anti-androgen compounds (such as enzalutamide, abiraterone acetate and apalutamide) [4,5,7,8]. What truly constitutes ‘standard systemic therapy’ is thus constantly debated. For surgical trialists, restricting men to historical agents with higher risks or greater side-effects may impact on acceptability of the study by patients and clinicians. This was the case in TroMbone, where the study was designed with ADT monotherapy and required a major amendment to permit additional systemic agents to enable accrual [16].
As such, all recruiting RCTs permit oncologists to use ADT with or without any systemic agent. To future-proof the SWOG 1802, all agents that are permitted by the National Comprehensive Cancer Network (NCCN) guidelines are permissible for use upfront. Patients in the IP2-ATLANTA trial undergo stratified randomisation at entry for systemic therapy use to ensure this remains representative across all arms.
There are issues with permitting all systemic agents, as head-to-head randomised comparison of the various systemic agents in mHSPC is lacking [2]. Sensibly, the majority of oncology trials and thus cRP studies use composite study endpoints (e.g. FFS, PFS) [1,24]. However, there is a risk that these subtle systemic therapy differences may impact upon the various individual components (e.g. biochemical, radiological) of these composite endpoints, generating potential for residual confounding. The magnitude of this effect is currently unknown.
Another challenge to clinical trialists is how to manage prostate EBRT for low volume patients in the SOC comparator arm, following the results of STAMPEDE Arm-H [12]. If men are presented with a ‘no local treatment’ versus ‘cRP’ study, the evidence from g-RAMPP’s early closure, would suggest this is not accepted by patients or clinicians. In contrast, the IP2-ATLANTA study permits low-burden patients only to have non-radical dose / fractionation prostate local radiotherapy, as per STAMPEDE Arm-H [12]. However, this can also be criticised for leading to confounders in comparing this arm to the other experimental arms. Nonetheless, with recent commissioning of EBRT for this group of patients, a surgical trial recruiting in this space cannot deny therapy within the SOC arm as a result. Attempting to compare the added value of an experimental intervention, such as cRP, against a moving standard of care background clearly remains a challenge.
Timing of surgery
All studies require systemic therapy to be commenced prior to surgery, although duration is not always defined. Historically, most patients have undergone at least six cycles of docetaxel, thus requiring a six to eight month period post-diagnosis to complete this treatment and then recover for surgery [1]. However, the minimum duration of time on other antiandrogen systemic agents required prior to local treatment, is not known.
As such, the SWOG 1802 study requires 22 to 28 weeks of SST prior to surgery. Similarly, the IP2-ATLANTA study mandates local treatment to occur at 32 (+/- 12) weeks post-study entry, irrespective of systemic agent choice [19]. The TroMbone study required patients to undergo surgery within 12-months from randomisation [16]. Finally, the SIMCAP trial requires >/= one month of ADT prior to surgery.
Surgical approach and use of extended pelvic lymph node dissection
There is limited evidence supporting superiority of any surgical approach in this setting [25]. The SIMCAP, IP2-ATLANTA, LoMP II and g-RAMPP studies permit open, laparoscopic or robotic-assisted approaches, as per treating surgeon.
The evidence supporting the added value of ePLND at the time of cRP in patients with distant disease is also unclear. In the case of g-RAMPP and TroMbone all patients underwent an ePLND. In IP2-ATLANTA, cRP + ePLND is recommended in all patients with nodal metastasis on baseline imaging and declared prior to randomisation. Thus, patients who undergo radiotherapy or minimally-invasive ablative therapies (HIFU / cryotherapy), instead of cRP, in an intervention arm would also undergo nodal radiotherapy or ePLND, respectively. In the SIMCAP trial ePLND is recommended, if surgically possible.
Multi-modal approach with metastasis-directed therapy
SWOG 1802 and IP2-ATLANTA permit the use of metastasis-directed therapy. The SWOG 1802 trial permits MDT for up to four metastatic sites prior to randomisation. The IP2-ATLANTA study allows patients with low-volume disease in intervention arms to undergo MDT to bone or nodal metastasis. This trial utilises either retroperitoneal lymph node dissection at time of cytoreductive prostatectomy and / or SABR directed MDT to bone and nodal disease following cRP.
Conclusion
In summary, despite adoption of cytoreductive prostate radiotherapy following the promising sub-group analysis of STAMPEDE (Arm-H) there remains a key window of opportunity to evaluate cytoreductive prostate surgery within the randomised controlled trial setting. The results of the aforementioned landmark studies will report on safety, feasibility and comparative oncological outcomes from cytoreductive prostate surgery in patients with de novo synchronous mHSPC. As such they will play a vital role in constructing new treatment pathways for patients diagnosed and deliver on the next important chapter as to whether there is indeed a role for cytoreductive prostate surgery within this treatment landscape.
References
1. Sweeney CJ, Chen Y, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New England Journal of Medicine 2015;373(8):737-46.
2. Marchioni M, Di Nicola M, Primiceri G, et al. New antiandrogen compounds compared to docetaxel for metastatic hormone sensitive prostate cancer: results from a network meta-analysis. The Journal of Urology 2020;203(4):751-9.
3. Vale CL, Fisher DJ, White IR, et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis. Annals of Oncology 2018;29(5):1249-57.
4. Connor MJ, Smith A, Miah S, et al. Targeting oligometastasis with stereotactic ablative radiation therapy or surgery in metastatic hormone-sensitive prostate cancer: a systematic review of prospective clinical trials. European Urology Oncology 2020;3(5):582‑93.
5. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. The Journal of Urology 2015;193(3):832-8.
6. Miyake H, Matsushita Y, Watanabe H, et al. Prognostic significance of time to castration resistance in patients with metastatic castration-sensitive prostate cancer. Anticancer Research 2019;39(3):1391-6.
7. Roubaud G, Liaw BC, Oh WK, Mulholland DJ. Strategies to avoid treatment-induced lineage crisis in advanced prostate cancer. Nature Reviews Clinical Oncology 2017;14(5):269.
8. Connor MJ, Shah TT, Horan G, et al. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nature Reviews Clinical Oncology 2020;17(3):168‑82.
9. Weichselbaum RR, Hellman S. Oligometastases revisited. Nature Reviews Clinical Oncology 2011;8(6):378.
10. Radwan N, Phillips R, Ross A, et al. A phase II randomized trial of Observation versus stereotactic ablative RadiatIon for OLigometastatic prostate CancEr (ORIOLE). BMC Cancer 2017;17(1):453.
11. Connor MJ, Winkler M, Ahmed HU. Survival in oligometastatic prostate cancer - a new dawn or the will rogers phenomenon? JAMA Oncology 2020;6(2):185-6.
12. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. The Lancet 2018;392(10162):2353-66.
13. Heidenreich A, Fossati N, Pfister D, et al. Cytoreductive radical prostatectomy in men with prostate cancer and skeletal metastases. European Urology Oncology 2018;1(1):46-53.
14. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. European Urology 2014;65(6):1058-66.
15. Sooriakumaran P, Karnes J, Stief C, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. European Urology 2016;69(5):788-94.
16. Sooriakumaran P. Testing radical prostatectomy in men with prostate cancer and oligometastases to the bone: a randomized controlled feasibility trial. BJU International 2017;120(5B):E8-E20.
17. Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. European Urology 2014;66(3):602-3.
18. Poelaert F, Verbaeys C, Rappe B, et al. Cytoreductive prostatectomy for metastatic prostate cancer: first lessons learned from the multicentric prospective local treatment of metastatic prostate cancer (LoMP) trial. Urology 2017;106:146-52.
19. Connor MJ, Shah TT, Smigielska K, et al. Additional treatments to the local tumour for metastatic prostate cancer-assessment of novel treatment algorithms (IP2-ATLANTA): protocol for a multicentre, phase II randomised controlled trial. BMJ Open 2021;11(2):e042953.
20. Connor MJ, Shah TT, Sukumar J, et al. Initial experience of the adjuvant treatments to the local tumor for metastatic prostate cancer: Assessment of novel treatment algorithms, a multicenter, phase II randomized controlled trial (IP2-ATLANTA). Journal of Clinical Oncology 2020
https://doi.org/10.1200/
JCO.2020.38.15_suppl.TPS5600
[accessed 30 September 2021].
21. Connor MJ, Edison M, Bass EJ, et al. PD34-12 MRI findings in de novo synchronous metastatic prostate cancer and implications for cytoreductive surgery. The Journal of Urology 2021;206(Supplement 3):e587.
22. Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multi-centre study. The Lancet 2020;395(10231):1208‑16.
23. Connor MJ, Dubash S, Bass E, et al. Clinical translation of avid metastases identified on prostate-specific membrane antigen (PSMA) PET/CT imaging in the management of de novo synchronous oligometastatic prostate cancer. European Urology Focus 2020;S2405-4569(20):30301-1.
24. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. The Lancet 2016;387(10024):1163-77.
25. Preisser F, Nazzani S, Mazzone E, et al. Comparison of open versus robotically assisted cytoreductive radical prostatectomy for metastatic prostate cancer. Clinical Genitourinary Cancer 2019;17(5):e939-45.
Declaration of competing interests: Martin J Connor is funded by the Wellcome Trust and University College London Hospitals (UCLH) Charity. Hashim U Ahmed’s research is supported by core funding from the United Kingdom’s National Institute of Health Research (NIHR) Imperial Biomedical Research Centre. He currently receives funding from the Wellcome Trust, NIHR (UK), Prostate Cancer UK, MRC (UK), Cancer Research UK, The Urology Foundation, The BMA Foundation, Imperial Healthcare Charity, Sonacare Inc., Trod Medical, and Sophiris Biocorp for trials in prostate cancer. He was also a paid medical consultant for Sophiris Biocorp, Sonacare Inc. and BTG in the past three years and currently a paid proctor for Sonacare and Boston.








