With an ageing population, the number of men being diagnosed with prostate cancer each year is steadily rising. With more specific investigations, such as multiparametric magnetic resonance imaging (MpMRI) and transperineal biopsies, the number of cases diagnosed at an earlier stage is also rising, leading to an increase in the numbers being treated.
Radical prostatectomy for prostate cancer is one of the mainstays of curative treatment, and although the incontinence rates after surgery have fallen with improved surgical technique, the higher volumes, younger demographic of patients and raised patient expectations have increased the number of patients presenting for treatment of their urinary incontinence. The increased use of salvage treatments for prostate cancer such as radiotherapy, high intensity focused ultrasound (HIFU) or brachytherapy, has lead to a greater patient cohort with urinary incontinence (UI).
UI is defined as “the involuntary loss of urine” by the International Continence Society (ICS), and stress urinary incontinence (SUI) is suggested if the involuntary leakage of urine occurs on exertion, sneezing or coughing.
The pathophysiology of post-prostatectomy urinary incontinence (PPI) is thought to be related to the sphincter muscles or bladder dysfunction or both. Bladder dysfunction can be in the form of detrusor overactivity or underactivity. Post prostatectomy incontinence is thought to be due to a number of reasons as the mechanism of urinary continence is complex. The urinary sphincter involves the inner smooth muscle and external striated rhabdosphincter, which control urethral closure pressure. The pelvic floor supports the sphincter and is comprised of the endopelvic fascia, levator ani muscles, arcus tendineus fascia, puboprostatic ligament and denovilliers fascia. The mobility and the position of the urethra as well as the nerve supply to all of the above play a role in male continence. Damage to any or all of these structures interoperatively is thought to contribute to PPI [1].
Urinary incontinence can be a difficult outcome to measure, with no set definition (i.e. completely dry / one pad a day). Some men may be actually dry but wear a pad for confidence. This lack of consensus on the definition has lead to widely different quoted incidence rates of post prostatectomy incontinence, ranging from 0.8% to 87% [2]. The reported rates also vary due to several different factors such as time after surgery when assessed and differences between surgeons’ and patients’ assessments. The same level of UI may not impact on the patients’ lifestyle in the same way in different individuals, which also leads to differences in self-reporting of UI. There are definitions for mild, moderate and severe UI, although not validated and universally accepted, but this can be helpful in deciding which treatment option is best (Table 1).
Table 1: Quantifying urinary incontinence.
Stamey Incontinence Score:
-
Mild: Only with severe stress.
-
Moderate: Minimal stress including walking.
-
Severe: Incontinence during bed rest.
Pads use:
-
Mild: one or two pads a day or <200ml/day.
-
Moderate: three to five pads a day or 200-500ml/day.
-
Severe: more than five pads a day or >500ml/day.
UI after prostatic surgery can be difficult to treat, and is a significant problem for the individual, impacting on multiple aspects of their life, including work, relationships and the financial burden of having to purchase continence products and loss of livelihood, which results in an overall lower quality of life (QoL). The healthcare costs for incontinence treatment are considerable as well.
Investigating male incontinence
The mainstay of investigating male UI is a full history including the cause of the incontinence (stress or urge), severity (number of pads used, size and estimate of volume) and voiding symptoms if any. Initial investigations would include urinalysis, a bladder diary, flow rate and post void residual. An incontinence questionnaire such as the ICIQ-SF, can be helpful to aid standardising of reported incontinence, pre- and post surgery, and to assess the impact on the patient’s life.
“UI after prostatic surgery can be difficult to treat, and is a significant problem for the individual, impacting on multiple aspects of their life including work, relationships and the financial burden of having to purchase continence products and loss of livelihood.”
If the incontinence persists and remains bothersome six months post prostatectomy in spite of conservative measures, a full re-evaluation is undertaken if a surgical option is to be considered. This requires a re-assessment of the severity of the incontinence with all the basic investigations and, most importantly, an assessment of the individual patient’s expectations and understanding of the options available.
Cystourethroscopy is recommended to evaluate sphincter damage and assess residual function and mobility, although its value is debatable. It also helps exclude any structural abnormality that may be exacerbating the problem, such as urethral or bladder neck strictures, bladder stones, tumour or diverticulum.
Urodynamics (UDS) are also recommended to help diagnose the type of incontinence, and to exclude detrusor overactivity or hypocontractility. Urodynamics may not always help predict the patient’s outcome post surgery, although it helps the choice of the appropriate procedure and facilitates better understanding by the individual patient who can therefore be counselled better. One study found that preoperative bladder dysfunction proven on UDS did not have a negative predictive value on post prostatectomy incontinence surgery when using an artificial urinary sphincter [3].
Management of urinary incontinence
The management of UI depends on the cause and the outcomes of the investigations. Patient choice, understanding, expectations, manual dexterity and performance status all influence the treatment option.
Pelvic floor therapy
The most widely recommended conservative treatment, and the initial recommended management option for patients with UI post prostatectomy, is supervised pelvic floor muscle therapy, with or without biofeedback. Some surgeons commence this prior to prostatectomy whilst others instigate pelvic floor therapy postoperatively. A recent meta analysis demonstrated that there was a significant reduced risk (36%), of postoperative incontinence at three months post radical prostatectomy if preoperative pelvic floor exercises where undertaken. However, preoperative pelvic floor muscle therapy did not make a difference in incontinence at one and six months postoperatively, suggesting that it hastens recovery time of incontinence postoperatively but does not reduce incontinence rates [4].
Pharmacological agents
There are no licensed pharmacological agents for use in men for stress urinary incontinence after prostatic surgery. However, duloxetine, a serotonin- noradrenalin reuptake inhibitor used for SUI in women, can be used off-licence in men with post prostatectomy SUI. Small studies have shown improvement in men with decreased usage of pads [5].
Detrusor overactivity causing overactive bladder symptoms can occur after radical prostatectomy, thought to be due to a mixture of partial decentralisation of the bladder, combined with somatic denervation and postoperative inflammation or infection. For overactive bladder symptoms, antimuscarinics and beta agonists are recommended. If these fail intravesical botulinum toxin is recommended [6].
Surgical treatment
The surgical options for proven SUI after post prostatectomy include urethral bulking agents, male urethral slings, artificial urinary sphincters and urinary diversion surgery.
Urethral bulking agents
There are several types of urethral bulking agents (silicone, collagen, autologous fat) that can be injected into the tissue surrounding the bladder neck via a cystoscope under local anaesthetic to improve continence. Although this option is less invasive than the other surgical techniques, the impact is short-lived with early failure rates as high as 50%, meaning repeated injections are needed. Multiple studies have shown bulking agents to be of limited value in SUI post prostatectomy [2,7,8]. Therefore they are only used in patients with mild post prostatectomy SUI [5,6].
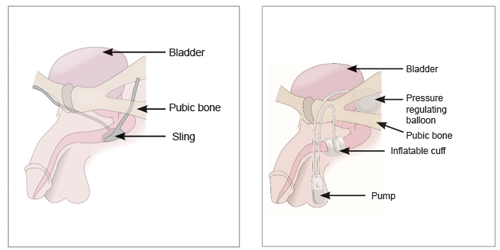
Figure 1 (left): Male synthetic sling. Figure 2 (right): Artificial urinary sphincter.
Male urethral slings
Male slings are placed via a perineal incision, somewhat similar to slings in females. In the case of the transobturator sling the mesh is placed on the urethral bulb, and the two lateral arms of the mesh are passed through the medial aspects of the obturator foramen, and pulled through the two smaller bilateral groin incisions (Figure 1). There are different theories as to how they work: a) repositioning the urethra to allow the external sphincter to work in the correct orientation as it was prior to surgery; b) by compressing the urethra. There are several different types of sling, including bone-anchored, adjustable and the transobturator slings, the latter being the most common. Newer slings including the Argus® and Remeex® are adjustable.
Complications include infection, urinary retention and thigh pain, and a number of small studies have confirmed their effectiveness [9]. Currently, male urethral slings are only recommend in the UK by the National Institute for Health & Care Excellence (NICE) if they are part of a trial, due to limited evidence showing the long-term outcomes [10]. At present the MASTERS trial, a multicentre national trial comparing the outcomes of male urethral slings to artificial urinary sphincters (AUS) for SUI in patients post prostatectomy surgery is underway to try and resolve some of these unanswered questions [11].
Artificial urinary sphincter
The AUS is the current gold standard of treatment for male urinary stress incontinence [10]. It consists of a cuff that is placed around the bulbar urethra, with a pressure regulating balloon in the prevesical space and a pump in the scrotum (Figure 2). When the patient wishes to urinate the pump is compressed manually which allows the cuff to deflate and urine to be passed through the urethra; the cuff subsequently reinflates spontaneously to close. This consequently requires adequate cognitive and hand function to use the device. The AUS is an invasive procedure, expensive and requires experienced surgeons to implant. It is also associated with several possible adverse outcomes including infection, erosion of the cuff and mechanical failure. The median life expectancy of the AUS is seven years, and changing the device generally becomes surgically more difficult with each change; this needs to be taken into consideration when treating younger patients [12]. The revision rates are as follows: due to mechanical failure – 8-45%; due to erosion / urethral atrophy and infection – 7-17% [5]. Some studies have shown its success rate to be up to 90% [13], but there is a wide variation of dry rates reported in the literature, varying from 4.3 to 85.7% [14], partially due to the lack of standardised definition of incontinence.
Several newer devices have recently been developed in an attempt to overcome the shortcomings of the AUS and preserve its proven efficacy. These include the FlowSecure™, which has two balloons to increase the sphincter pressure when intra-abdominal pressure rises, and the ZSI 375 (Zephyr) device which has the pump and the pressure regulating fitted within the scrotum. However, the AMS 800™ remains the most commonly used artificial urinary sphincter [14].
Urinary diversion
Urinary diversion may be considered in patients when the urethra is unusable, such as with complex urethral strictures, or when all other options have failed. Other options include bladder neck closure with a continent catheterisable channel (Mitroffanaff) or an ileal conduit. Both involve major surgery with associated risks, and both have significant long-term complications. However, if the incontinence is causing a major impact on the patients QoL, then they may need to be considered.
Factors that influence the choice of operation for the patient
For over four decades the AUS has been the mainstay of surgical treatment for PPI, with success rates of up to 90% [15]. However, due to relatively high complication rates, including infection, cuff erosion and mechanical failure there has been much research into alternative treatment options, including several different types of male urethral sling. Until the results of head-to-head trials are known, the use of alternative devices remain dependent on individual expertise and experience.
When deciding on which operation for which patient several factors need to be considered: patient preference based on a detailed understanding of the options available, time elapsed post prostatectomy, degree of incontinence and burden of symptoms, prior radiation and prior incontinence surgery. The patients’ cognition and understanding of the risks and benefits of the treatment options has to be weighed up against their need to be continent to improve their QoL. Hand function is very important as, if they wish an AUS to be placed, they need to be able to have the dexterity to use the pump, as well as have the motivation to learn how to.
Although diagnostic tests cannot predict outcomes, they certainly can help towards the decision-making process. Urethrocystoscopy is proposed as useful in helping decide between the AUS and a sling, as it can be used to perform a ‘repositioning test’, and evaluate the patient’s residual sphincter function including the functional sphincter length, thereby determining the patients suitability for a retrourethral transobturator sling [16]. It can also diagnose other pathologies that may impact upon a patient’s suitability for surgery, such as bladder diverticulum, stones or tumours, bladder neck stenosis or urethral stricture disease, all of which would change the management of the patient. Similarly, urodynamic findings on detrusor overactivity, poor compliance, or significantly reduced functional bladder capacity, will influence the choice of surgery.
“The patients’ cognition and understanding of the risks and benefits of the treatment options has to be weighed up against their need to be continent to improve their QoL.”
One study showed that the two main predictive values for better surgical outcomes were: less severe UI preoperatively and length of time from prostatectomy until UI surgery. Patients with mild-moderate UI preoperatively had better continence outcomes and the longer the time between the two surgeries, the better the outcome [3].
Several studies have reported that when patients are given a choice, even if they were advised to have the AUS, the majority opted to have a sling [15].
Specific patient factors such as degree of incontinence and prior radiation have an impact on the type of surgical treatment chosen.
Transobturator slings have been shown in a number of publications to be less effective if the patient’s incontinence is severe. With mild to moderate incontinence, good outcomes have been reported with the male urethral sling, but in small studies. If the UI is severe then the AUS may be the preferred option. Prior radiation is a significant negative prognostic factor on urethral sling outcomes, and the sling is not recommended in these patients [16]. This is thought to be due to lack of urethral mobility after radiation; as the sling works by proximal urethral relocation, the urethra needs to be mobile enough to achieve this. Consequently in men with PPI who have had radiotherapy the AUS is the recommended treatment option.
Prior incontinence surgery also effects the treatment choice. If a patient has had a prior AUS, subsequent sling placement generally does not change the incontinence rate due to the urethra becoming fibrotic and non-compliant after AUS surgery. However, in contrast, replacing an existing AUS device with a new urethral cuff, such as downsizing, repositioning or placing a double cuff has improved continence outcomes.
If a patient has had a prior sling, there is a fairly high failure rate in continence with repeat sling surgery. However, if the sling was transobturator in nature, a placement of an AUS device after this is not surgically complicated, and has been shown to have high success rates [15].
Due to the fact that slings are less invasive than the AUS procedure this could mean a wider cohort of patients, with a wider range of co-morbidities able to be considered for incontinence surgery. Also, as it has no mechanical component, men do not need the same level of manual dexterity that they do to operate the AUS device.
Conclusion
Urinary incontinence remains a significant problem following treatment for prostate cancer, which significantly impacts on QoL and healthcare costs. Currently, the gold standard of treatment is the AUS [10]. In selected patients small studies have shown that male urethral slings may offer an alternative to the AUS. There is currently no randomised controlled trial comparing the outcomes of all the current surgical treatment options for PPI. The MASTERS trial which is underway at the moment seeks to correct this; it is a randomised multicentre study comparing the male synthetic sling to the artificial urinary sphincter in men with urodynamic stress urinary incontinence after prostatic surgery. The primary outcomes are the clinical effectiveness of implanting the male sling versus the AUS in terms of patient reported incontinence at 12 months, and the cost-effectiveness measured in quality adjusted life years at 24 months [11].
References
1. Heesakkers J, Farag F, Bauer RM, et al. Pathophysiology and contributing factors in postprostatectomy incontinence: a review. Eur Urol 2016; [Epub ahead of print].
2. Silva LA, Andriolo RB, Atallah ÁN, da Silva EMK. Surgery for stress urinary incontinence due to presumed sphincter deficiency after prostate surgery (Review). The Cochrane Library 2014;9.
3. Holm HV, Fosså SD, Hedlund H, et al. Severe post prostatectomy incontinence: Is there an association between preoperative urodynamic findings and outcome of incontinence surgery? Scand J Urol 2015;49(3):250-9.
4. Changa J, Lamb V, Patelc MI. Preoperative pelvic floor muscle exercise and postprostatectomy incontinence: a systematic review and meta-analysis. European Urology 2016;69(3):460-7.
5. Bauer RM, Gozzi C, Hübner W, et al. Contemporary management of postprostatectomy incontinence. European Urology 2011;59(6):985-96.
6. Thiruchelvam N, Cruz F, Kirby M, et al. A review of detrusor overactivity and the overactive bladder after radical prostate cancer treatment. BJU Int 2015;116:853-61.
7. Secin FP, Martinez-Salamanca JI, Eilber KS. Limited efficacy of permanent injectable agents in the treatment of stress urinary incontinence after radical prostatectomy. Archivos Espanoles de Urologia 2005;58(5):431-6.
8. Imamoglu MA, Tuygun C, Bakirtas H, et al. The comparison of artificial urinary sphincter implantation and endourethral macroplastique injection for the treatment of post prostatectomy incontinence. European Urology 2005;47:209-13.
9. Welk BK, Herschorn S.The male sling for post-prostatectomy urinary incontinence: a review of contemporary sling designs and outcomes. BJU Int 2012;109(3):328-44.
10. NICE. Lower urinary tract symptoms in men: management (Clinical guideline CG97).
www.nice.org.uk/guidance/CG97/chapter/
1-Recommendations
#surgery-for-voiding-symptoms-2
Accessed November 2016.
11. www.mastertrial.co.uk
Accessed November 2016.
12. Venn SN, Greenwell TJ, Mundy AR. The long term outcome of the artificial urinary sphincter. J Urol 2000;164:702-7.
13. Herschorn S. The artificial urinary sphincter is the treatment of choice for post–radical prostatectomy incontinence. Can Urol Assoc J 2008;2(5):536-39.
14. Van der Aa F, Drake MJ, Kasyan GR, et al. The artificial urinary sphincter after a quarter of a century:a critical systematic review of its use in male non-neurogenic incontinence. European Urology 2013;63(4):681-9.
15. Comiter CV, Dobberfuhl AD. The artificial urinary sphincter and male sling for post prostatectomy incontinence: Which patient should get which procedure? Investig Clin Urol 2016;57(1):3-13.
16. Kretschmer A, Hübner W, Sandhu JS, et al. Evaluation and management of postprostatectomy incontinence: a systematic review of current literature. European Urology Focus 2016;2(3):245-59.
TAKE HOME MESSAGE
-
Multimodality treatment for prostate cancer has increased post treatment incontinence.
-
Urinary incontinence has a high impact on QoL.
-
Urodynamics are important to rule out causes of incontinence other than sphincter incompetence, but do not predict success of surgery.
-
AUS is currently the ‘gold standard’ of treatment for PPI.
-
Male urethral slings are emerging as an important alternative to the AUS.
-
The MASTERS trial is in progress and will compare AUS to male urethral sling outcomes.
Useful websites
www.mastertrial.co.uk
www.nice.org.uk
www.ics.org
https://uroweb.org/guideline/urinary-incontinence
Declaration of competing interests: None declared.