Artificial intelligence (AI) – “the mimicking of human cognition by computers” – is a rapidly expanding field within medicine [1,2]. There is increasing evidence that AI may enhance the delivery of healthcare [1]. A well-known example is an AI system known as ‘Watson’ created by IBM which is a decision support tool towards the diagnosis and management of oncology patients at Memorial Sloan Kettering Cancer Center [1].
As practitioners of medicine, we spend a lifetime refining our intuition with hard earned knowledge and skills to acquire the mindset for sophisticated decision-making in our increasingly complex patients. However, Watson can pull information and make decisions based on millions of such human physicians’ life-time experiences, medical reports, patient records, clinical trials and published literature [1]. So, will Watson surpass a seasoned human medic? Could Watson replace the human doctor in the future? This article attempts to answer these questions and place into perspective the burgeoning field of AI as it is relevant to urology.
Technical aspects of AI: a primer for the clinician
Data analytics and AI are contemporary hot topics which transcend disciplines and transform diverse sectors, making an increasingly significant impact on medicine.
AI is a broad term that designates a variety of fields and techniques in computer science. It seeks to develop computer algorithms (a mathematical set of rules) to accomplish tasks traditionally associated with human intelligence, such as the ability to learn and solve problems.
Two key sub-types of AI that need defining are machine learning (ML) and deep learning (DL). ML seeks to provide knowledge to computers through data and observations without being explicitly programmed. DL goes a step further and uses learning that relies on multiple processing layers (hence, deep) of data, typically a few million data points.
Typically, in a clinical application, the aim of an AI system is to capitalise on the raw data, frequently in the form of images or numbers e.g. results from clinical tests. The raw data may comprise different variables both in terms of origin (e.g. demographic information, pathology, radiology and self-reported symptoms) and types (binary, categorical and continuous variables).
Both ML and DL systems are then used to process this raw data. An iterative and complex mathematical process using high computational power then processes this raw data (input) and turns it into clinically relevant knowledge (output). This acquired knowledge is akin to what higher human intelligence would have produced. This high quality of clinical intelligence is then made accurate enough so it can be applied directly to various patient processes and decision-making with minimal errors.
To achieve this high level of accuracy, the most critical aspect is to optimise the ‘goodness of fit’ between the input and output. High levels of ‘goodness of fit’ can only be obtained when the ML and DL systems are fed large amounts of variegated data, which is also known as ‘big data’. This complex, large and variable data is required because AI is trying to simulate the following:
- A large variation in human pathophysiology.
- The collective wisdom of many expert clinicians who have assimilated large amounts of anecdotal and evidence based knowledge in their lifetimes.
Finally, the trained model is tested on previously unseen data to demonstrate its validity, i.e. the true outcome is withheld, and compared against what the AI system would estimate.
A high-level illustration of an application using AI in medicine is provided in Figure 1. For a more in-depth technical overview we refer readers to standard authoritative texts, for example Bishop and Hastie et al. [3,4].
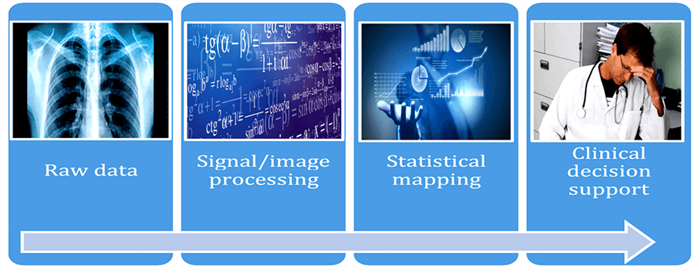
Figure 1: Key components in the AI workflow to develop clinical decision support tools.
The now: current status of AI in urology
In the UK, prostate cancer is the most common cancer in males, with around 130 new cases diagnosed each day [5]. Artificial neural networks (ANN) were developed to mimic the ability of the human brain to learn from personal experience. Evidence shows that ANN could significantly aid the radiological diagnosis of prostate cancer [6]. In 1999, a study by Loch and colleagues found that using artificial neural network analysis (ANNA) significantly improved the accuracy of prostate cancer identification on transrectal ultrasound (TRUS) [7]. Tokas et al. found that the combination of ANNA and computerised TRUS produced a high level of accuracy (97%) when detecting prostate cancer [8]. A study by Lee et al. found that a support vector machine (SVM) approach could accurately predict prostate cancer based on basic biographic and laboratory data as well as TRUS results [9]. Thompson et al. reported an impressive sensitivity of 96% on multi-parametric magnetic resonance imaging (mpMRI) for detection of prostate cancer, however specificity was a considerably lower 36% [10].
Importantly, the outcome of mpMRI with the Prostate Imaging Reporting and Data System (PI-RADs) is highly variable depending on the radiologist as demonstrated in a study published by Sonn and colleagues [11]. Liu and colleagues demonstrated better specificity and sensitivity when classifying prostate cancer lesions on MRI using XmasNet, a newly developed deep convolutional neural network (CNN), compared to other conventional machine learning models [12]. Ishioka and colleagues developed a computer-aided diagnosis system using deep learning architecture which showed great potential when classifying prostate lesions on MRI [13]. These studies highlight that AI could transform prostate cancer diagnostics and is likely to become an integral part of diagnostic algorithms [10,13].
The use of AI in the radiological diagnosis of other urological cancers has also been explored. Kunapuli and colleagues found that the use of ensemble and relational machine-learning methods significantly improved the classification of renal masses on CT imaging [14]. Certain lesion characteristics such as texture and shape are associated with malignant tumours [14]. This study developed AI frameworks based on the texture metrics of renal tumours derived from CT imaging and found that diagnostic accuracy significantly improved [14].
“Rather than replace urologists, AI will mainly be used to inform, enhance, and complement their practice.”
Granter et al. were among the first to review recent studies that showed comparable performance of AI to those of experienced pathologists [15]. An editorial on this paper by Sharma et al. concluded by stating that AI technologies are best seen as synergistic to human cognition and that the question of human against a computer was more a question of a human versus human with the computer [16]. In uro-pathology, Guo and colleagues broke new ground at the European Association of Urology (EAU) meeting in 2018 when they demonstrated in a pilot study that AI could perform Gleason scoring of prostate cancer with accuracy levels comparable to that of a human pathologist [17]. In 2016, Cosma and colleagues developed a neuro-fuzzy based model which was more accurate at predicting pathological stage of prostate cancer compared to the older AI models with an area under the curve of 0.812 [18].
Jena, an oncologist at Addenbrooke’s hospital in Cambridge, recently presented his work using AI to plan for radiotherapy treatment for prostate cancer. Planning involves defining tumour and normal tissue boundaries with great precision. This process can take hours. With AI, this painstaking task can be completed in minutes. Deep Decision Forests, a type of DL algorithm, was used to perform planning of radiation treatment to the prostate. The accuracy of this to outline normal and abnormal tissue was as good as the radiation oncologist [19].
There is also the concept of combining AI with virtual reality, perhaps through an avatar or chatbots? The use of avatars in healthcare services is still in the early research stages, however various models have been developed [20]. For example, ‘Fredrick’ is a mobile medical avatar created to help diabetic patients with daily routines such as blood sugar monitoring [20]. Could an intelligence enabled medical avatar run a prostate cancer follow-up clinic via a clinical decision support system?
In 2014, Lam and colleagues developed an ANN to predict five-year mortality following radical cystectomy for invasive bladder cancer which had an accuracy of 77.8% [21]. An ANN model created by Ajili et al., demonstrated a sensitivity of 96.66% and specificity of 100% at predicting response to bacillus calmette guerin (BCG) therapy in non-muscle invasive bladder cancer [22].
As shown there are vast amounts of research looking into the application of AI in urological cancer management, however there are also studies looking into other urological conditions. For example, Barzegari and colleagues developed an ANN model to predict urethral pressure in patients which could be used to help urologists diagnose and manage stress incontinence [23]. Goyal and colleagues, discussed an ANN which could determine the number of shocks and shock power needed when treating renal stones with extracorporeal shock wave lithotripsy (ESWL) [24].
The future: impact of AI on urological practice
As AI inevitably becomes part of our day to day work, urology as a speciality will change. Trainees will be faced with a rapidly changing way of working. AI is already being used to mine the ongoing plethora of evidence-based medicine. By integrating this into the electronic health record, the relevant aspects of the most up to date evidence can be brought into play during the clinician-patient encounter in real time. Computers will soon act as ‘clinical decision makers’. Conventional clinical examination will become extinct, as information from portable ultrasound devices together with patient entered data seamlessly integrate into the electronic healthcare record [25]. However, communication, compassion, empathy and caring will continue to be crucial interpersonal skills. High level computation is the forte of AI whilst high level cognition will remain a human forte. Thus, by taking up some of the more logical, computable aspects of care, AI will release clinicians to focus more on the patient-doctor relationship [25]. Rather than replace urologists, AI will mainly be used to inform, enhance, and complement their practice. This will allow the urologist to focus on the more complex aspects of the patient-disease intersection. Thus, from this perspective, ‘Artificial Intelligence’ may be better called ‘Augmented Intelligence’. An article by Jha and Topol discusses the concept of radiologists and pathologists merging into one speciality called ‘information specialists’ who would work in tandem with AI [2].
The future: impact of AI on urological training
Health Education England recently published an interim report on The Topol Review which identified genomics, digital medicine, AI and robotics as the key technologies that will herald the fourth industrial revolution in healthcare [25]. Clinicians will need to continually update their knowledge and update skills to keep up with the technology [25]. Doctors should be actively involved in the development and integration of these new technologies into current practice [25]. Furthermore, clinicians must be given appropriate time and support when learning to use new programmes [25]. As well as training clinicians to use AI and other novel technology, there is ongoing development surrounding clinical informatics as a subspecialty [26].
In 2016, clinical informatics became the latest sub-speciality in the United States and there are now board certified, two-year fellowships after completion of residency in any field of medicine or surgery. In the future, consultant urologists could have a sub-specialist interest in clinical informatics. Clinical informatics fellowships may become commonplace with urology registrars taking up to two years out of training post FRCS(Urol). However, considering the huge impact AI is likely to have on patient care, there should be education and training incorporated throughout medical school, foundation training and into core training. Figure 2 illustrates our theoretical timeline of the future urologist with an interest in clinical informatics.
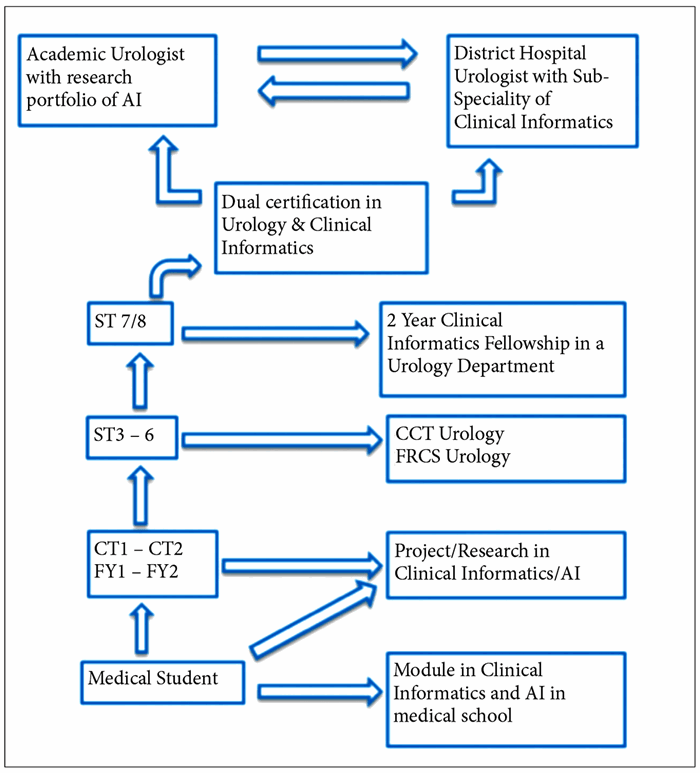
Figure 2: The Future Urologist & Training in AI.
Conclusion
The current literature on AI in urology remains mainly in the realm of ANN, which has been around for decades [7]. Studies using machine learning of big clinical datasets are only starting to emerge. Also, there is a limited number of studies which validate the use of an AI system in clinical practice. Thus, the clinical evidence to prove the true transformative potential of AI is at present limited.
But, now advances in ‘Big Data’, vastly increased computational power and economies of scale from cloud computing are the three factors transforming AI by enabling machine learning to humanoid level capabilities. Thus, the field is now ripe for considerable breakthroughs. We envisage AI may lead to improving diagnosis and even prognosis of urological disease, provide novel insights into disease symptom trajectories, determine disease subtypes, and ultimately assist in the quest of developing personalised solutions in our clinical practice.
However, as with all such technological advances in healthcare, the ‘people’ factor is lagging behind. It is now incumbent upon the urology community to grasp this mantle and provide the leadership to drive AI in our hospitals, and to integrate it into our workflows and clinical processes. If we are to harness these opportunities in urology, we need to start breaking down the traditional boundaries between disciplines and collaborate in a truly interdisciplinary way with data scientists and industrial partners. Data scientists need to deeply understand the daily practical problems urologists face, from meeting patients and data collection to decision-making. It is up to us, especially those in larger academic centres, to make this an integral part of our research endeavour, and provide the clinical evidence to back it. Only with such zeal, and intense collaboration, will we be able to usher in a new era of care for our patients.
References
1. Diprose W, Buist N. Artificial Intelligence in medicine: humans need not apply? NZMJ 2016;129:73-6.
2. Jha S, Topol EJ. Adapting to artificial intelligence radiologists and pathologists as information specialists. JAMA 2016;316(22):2353-4.
3. Bishop CM. Pattern recognition and machine learning. Springer; 2007.
4. Hastie T, Tibshirani R, Friedman J. The elements of statistical learning: data mining, inference, and prediction, 2nd ed. Springer; 2009.
5. Cancer Research UK.
www.cancerresearchuk.org/
health-professional/cancer-statistics/
statistics-by-cancer-type/
prostate-cancer#heading-Zero
6. Ghaderzadeh M, Fein R, Standing A. Comparing performance of different neural networks for early detection of cancer from benign hyperplasia of prostate. Appl Med Inform 2013;33(3):45-54.
7. Loch T, et al. Artificial neural network analysis (ANNA) of prostatic transrectal ultrasound. Prostate 1999;39(3):198‑204
8. Tokas T, Grabski B, Paul U, et al. A 12-year follow-up of ANNA/C-TRUS image-targeted biopsies in patients suspicious for prostate cancer. World J Urol 2018;36(5):699-704.
9. Lee HJ, Hwang SI, Han SM, et al. Image-based clinical decision support for transrectal ultrasound in the diagnosis of prostate cancer: comparison of multiple logistic regression, artificial neural network, and support vector machine. Eur Radiol 2010;20(6):1476-84.
10. Thompson JE, van Leeuwen PJ, Moses D, et al. The diagnostic performance of multiparametric magnetic resonance imaging to detect significant prostate cancer. J Urol 2016;195(5):1428-35.
11. Sonn GA, Fan RE, Ghanouni P, et al. Prostate magnetic resonance imaging interpretation varies substantially across radiologists. Eur Urol Focus 2017;S2405-4569(17):30266-3.
12. Liu S, Zheng H, Feng Y, Li W. Prostate cancer diagnosis using deep learning with 3D multiparametric MRI. Proceedings of SPIE Medical Imaging, 2017, Orlando, Florida, United States.
www.spiedigitallibrary.org/
conference-proceedings-of-spie/
10134/1013428/Prostate-cancer-diagnosis
-using-deep-learning-with-3D
-multiparametric-MRI/
10.1117/12.2277121.short?SSO=1
13. Ishioka J, Matsuoka Y, Uehara S, et al. Computer-aided diagnosis of prostate cancer on magnetic resonance imaging using a convolutional neural network algorithm. BJU Int 2018;122(3):411-17.
14. Kunapuli G, Ganapathy P, Varghese B, et al. MP63-10 development of a clinical decision-support tool for classification of renal masses. J Urol 2018;199(4):844-5.
15. Granter SR, Beck AH, Papke Jr DJ. AlphaGo, deep learning and the future of the human microscopist. Arch Pathol Lab Med 2017;141(5):619-21.
16. Sharma G, Carter A. Artificial intelligence and the pathologist: future frenemies? Archives of Pathology & Laboratory Medicine 2017;141(5):622-3.
17. Zhang C, Zhang Q, Gao X, et al. High accuracy and effectiveness with deep neural networks and artificial intelligence in pathological diagnosis of prostate cancer: initial results. European Urology Supplements 2018;17(1):e304-8.
18. Cosma G, Acampora G, Brown D, et al. Prediction of pathological stage in patients with prostate cancer: a neuro-fuzzy model. PLOS ONE 2016;11(6):e0155856.
19. Jena R. accelerating radiation oncology with cloud-based artificial intelligence. Lecture at University of Cambride, UK. November 2018.
www.cuh.nhs.uk/sites/default/
files/news/Medicine%20for%
20members%20talk%2007-11-18.pdf
20. Shaked NA. Avatars and virtual agents - relationship interfaces for the elderly. Healthc Technol Lett 2017;4(3):83-7.
21. Lam KM et al. Using artificial neural network to predict mortality of radical cystectomy for bladder cancer. Smart Comp 2014;201-7.
22. Ajili F, Mhamed Issam B, Abid S, et al. Prognostic value of artificial neural network in predicting bladder cancer recurrence after BCG immunotherapy. J Cytol Histol 2014;5:3.
23. Barzegari M, Vahidi B, Reza Safarinejad M, Hashemipour M. Pathological analysis of stress urinary incontinence in females using artificial neural networks. Cornell University; 2018. https://arxiv.org/abs/1803.01843
24. Goyal NK, Kumar A, Trivedi S, et al. A comparative study of artificial neural network and multivariate regression analysis to analyze optimum renal stone fragmentation by extracorporeal shock wave lithotripsy. Saudi J Kidney Dis Transpl 2010;21(6):1073-80
25. Health Education England. The Topol Review. Preparing the healthcare workforce to deliver the digital future. Interim Report. Health Education England. ; 2018.
26. Gardner RM, Overhage JM, Steen EB, et al. Core content for the subspecialty of clinical informatics. Journal of the American Medical Informatics Association 2009;16(2):153-7.
Declaration of competing interests: None declared.





