A 72-year-old gentleman is referred to you in the two-week wait clinic with a prostate specific antigen (PSA) of 22ug/L. He is otherwise fit and well and does not take any regular medication. His multi-parametric magnetic resonance imaging (mpMRI) shows a Prostate Imaging Reporting and Data System (PIRADS) 5 lesion, T3bNxMx, which is confirmed to be Gleason 4+4 adenocarcinoma of the prostate on transperineal biopsy.

Figure 1.

Figure 2.

Figure 3.
- How would you risk-stratify this patient using the Cambridge Prognostic Group (CPG) classification, assuming he has no skeletal metastases or pelvic lymphadenopathy?
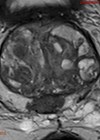
- he patient undergoes further imaging as part of staging his disease. What type of scan is shown in Figure 1 and what does it show? How sensitive and specific is this modality?
- What management options are available for this patient?
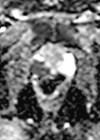
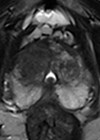
- What type of scan is shown in Figures 2 and 3? What are the indications for this modality in the context of prostate cancer? What do the images show? What is its sensitivity and specificity?
Answers
1. The Cambridge Prognostic Group (CPG) classification system is a five-tiered system that replaced the original three-tiered system [1]. This patient is in CPG5 as he has more than two of the following criteria: PSA >20ug/L and / or Gleason 4+4 and / or T3 disease. It is important to note that we do not yet know our patient’s lymph node and metastases status and so CPG is provisional.
2. This is an isotope whole body bone scan which uses Technetium-99 (99mTc) tracer to demonstrate high bone activity such as in bone metastases. For the patient in this case, there are several areas of increased tracer uptake throughout the skeleton corresponding to extensive bone metastases. There is no tracer uptake in the kidneys, a finding known as ‘Super Scan’, due to the extensive osteoblastic metastases causing diminished renal and background soft tissue uptake. Bone scans are indicated in prostate cancer for patients who are CPG3 and above, any concern for bony metastases and patients on watchful waiting with a high risk of bony metastases [2]. The reported sensitivity is 79% and specificity is 82% [3].
3. The management of prostate cancer with bone metastases at presentation would include androgen deprivation therapy (ADT), which could be in the form of surgical or hormonal castration. National Institute for Health & Care Excellence (NICE) guidelines also recommend that if the patient has good performance status, they should also be given docetaxel chemotherapy. This was based on the STAMPEDE trial arms, where they delivered six three-weekly cycles, which showed an overall increased survival benefit with docetaxel (59.1 months) versus long-term ADT alone (43.1 months) [4]. Patients may also be offered radiotherapy for disease and symptom control (rather than cure) if they have low metastatic burden [6].
4. This is a prostate-specific membrane antigen-positron emission tomography (PSMA-PET) scan. Figure 2 is a maximum intensity projection (MIP) image showing PSMA uptake in the prostate, internal iliac lymph nodes and skeletal metastases. Figure 3 is an axial fused PET-CT image demonstrating uptake in the left seminal vesicle, a left internal iliac lymph node and the left superior pubic ramus.
PSMA is a membrane glycoprotein that is expressed at 100–1000-fold higher levels in prostate cancer as compared with healthy prostate tissue. PSMA PET scans use molecules (ligands) with radioactive tracers that specifically bind to PSMA enabling rapid accumulation in viable prostate cancer cells. There are several ligands including Galium-68 (68Ga), Fluorine-18 (18F) and Piflufolastat F-18 (18F-DCFPyL).
This overexpression of PSMA glycoprotein in prostate cancer cells is stipulated to be better at identifying lymph node and bone metastases than other imaging modalities. However, PSMA can also be expressed in other conditions thus sensitivity is 89–97% but specificity is reported around 40–56% [3]. The indications for PMSA-PET scans as per the European Association of Urology (EAU) guidelines include initial staging in intermediate and high-risk prostate cancer if available [3], and for suspected prostate cancer recurrence.
References
1. Parry MG, Cowling TE, Sujenthiran A, et al. Risk stratification for prostate cancer management: value of the Cambridge Prognostic Group classification for assessing treatment allocation. BMC Med 2020;18:114.
2. NICE. Guideline NG131: Prostate cancer: diagnosis and management. NICE; 2019
https://www.nice.org.uk/guidance/ng131
3. Cornford P, Tilki D, Van Den Bergh RCN, et al. EAU Guidelines on Prostate Cancer 2024. EAU Guidelines Office; Arnhem, The Netherlands; 2024.
https://uroweb.org/guidelines/
prostate-cancer/diagnostic-evaluation
4. Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol 2019;30(12):1992–2003.
5. Naik M, Khan SR, Lewington V, et al. Imaging and therapy in prostate cancer using prostate specific membrane antigen radioligands, British Journal of Radiology, 2024;97(1160):1391–404
6. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. The Lancet 2018;392(10162):2353–66.
7. Djaïleb L, Armstrong WR, Thompson D, et al. Presurgical 68Ga-PSMA-11 Positron Emission Tomography for Biochemical Recurrence Risk Assessment: A Follow-up Analysis of a Multicenter Prospective Phase 3 Imaging Trial. Eur Urol 2023;84(6):588–96.
[All links last accessed June 2025]