You are referred a 68-year-old gentleman to the rapid access prostate clinic with a serum prostate specific antigen (PSA) of 12ug/L. He is otherwise fit and well with mild voiding lower urinary tract symptoms (LUTS). He undergoes a multi parametric MRI scan (mp-MRI).

Figure 1.

Figure 2.

Figure 3.

Figure 4.

Figure 5: Material provided courtesy of Boston Scientific.
© 2025 Boston Scientific Corporation or its affiliates. All rights reserved.
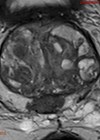
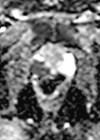
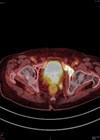
1. What are the mp-MRI sequences in figures 1–4 and where is the lesion?
2. The patient undergoes a transperineal biopsy. Histology confirms Gleason 4+3 adenocarcinoma of prostate, 12/18 cores positive, pattern 4 greater than 20%, therefore ISUP grade group 3. What are the treatment options for localised prostate cancer with a Gleason grade of 4+3 (Grade Group 3)?
3. How does the Prostate Testing for Cancer and Treatment (PROTECT) Trial influence treatment decisions in localised prostate cancer?
4. He is seen in the clinic after multidisciplinary team (MDT) and offered radical treatment options. What are the risks and outcomes associated with radical treatment options?
5. What is seen in figure 5 and how does it work?
Answers
1 .Image 1 is the axial T2-weighted image of a PI-RADS 5 mid-gland lesion in the right transition zone. The lesion appears dark, measuring 19mm in diameter and would be T2bN0M0. Image 2 and image 3 is the same lesion demonstrated on the high b value (b1400) Diffusion weighted image (DWI) and the apparent diffusion coefficient (ADC) map respectively. Image 4 shows the same lesion with increased contrast uptake on the early dynamic contrast enhanced sequence (although contrast has limited role in the evaluation of the transition zone) [1].
2. For a patient with localised prostate cancer, treatment options include radical prostatectomy (robotic-assisted laparoscopic prostatectomy – RALP), radiotherapy (external beam (EBRT) or brachytherapy), and hormonal therapy as an adjunct. The decision depends on cancer staging, patient health, and personal preferences [2].
Active surveillance is most appropriate for low-risk prostate cancer (Gleason ≤6, prostate specific antigen (PSA) <10). The National Institute for Health & Care Excellence (NICE) suggests the active surveillance protocol for year one should involve three to four monthly PSA, personal PSA thresholds, digital rectal exam (DRE) at 12 months, MRI at 12–18 months, biopsy if PSA or MRI changes [3]. Active surveillance would not be considered best practice for our patient unless they choose not to have radical treatment.
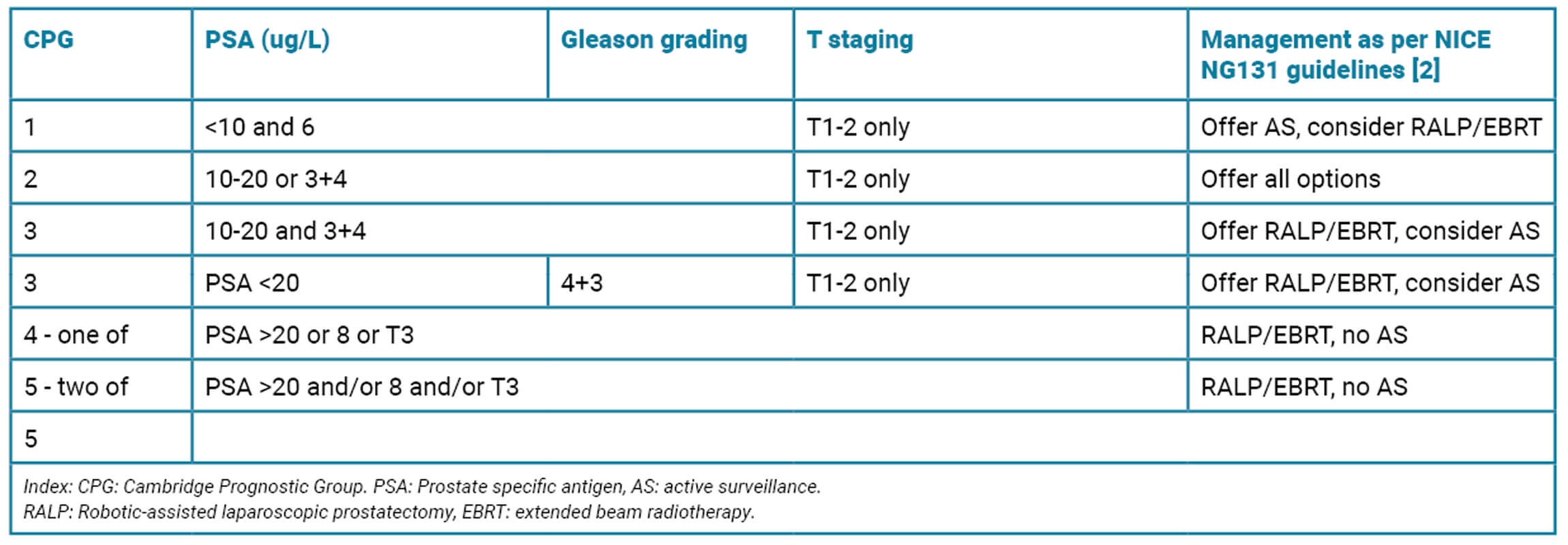
3. PROTECT was a randomised controlled trial which compared active surveillance (AS), RALP and RT (radical radiotherapy – external beam or brachytherapy) in men with localised prostate cancer. Over 10 years, prostate cancer-specific survival was high across all groups (>96.9%). However, the rates of metastasis were lower with surgery or radiotherapy (4.7% in RALP vs. 5% in RT vs. 9.4% in the active monitoring group). Clinical progression occurred in 25.9% in AS vs. 10.5% RALP vs. 11% in RRT. This suggests that while active surveillance (AS) is viable in lower-risk patients, definitive treatment may be more appropriate in intermediate-risk disease [4].
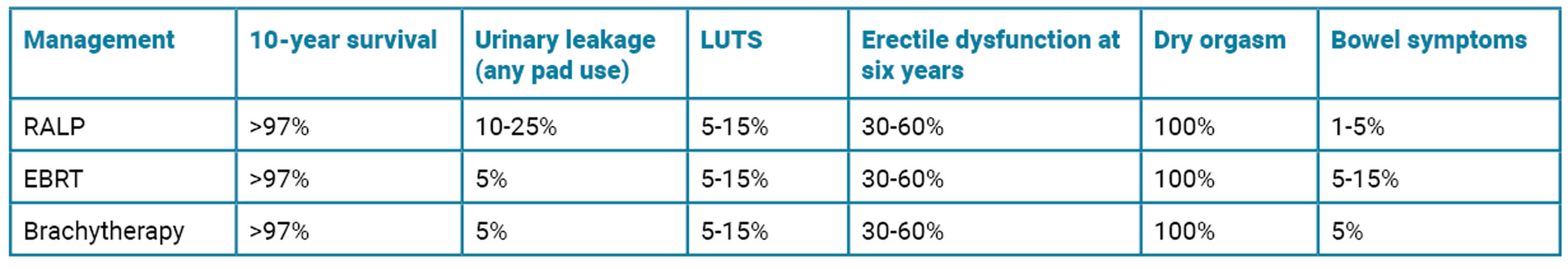
4. When counselling a patient on radical treatment options it is important to explain the risks and recovery afterwards, in addition to fertility, sexual function, continence and lower urinary tract symptoms. The risks are summarised in the below table (PROTECT [4], CHIPP trial [5] and UK National Prostate cancer audit). RALP also has associated risks of anastomotic leak, stricture and bladder neck stenosis. RALP and brachytherapy have associated GA risks. Radiation cystitis can occur many years later.
5. This is an artificial urinary sphincter (AUS) offered for male urinary incontinence as might be seen after radical prostatectomy. There is a cuff that sits around the bulbous urethra which can be inflated and deflated by the pump in the scrotum. The water filled reservoir is usually placed in the retropubic space. To pass urine the cuff is deflated and automatically re-inflates after five minutes to stop flow [6].
References
1. Sultana A, Tam H, Ahmed HU, Leung LY. Prostate cancer series: diagnostics 1. Urology News 2025;29(2):17–8.
2. NICE. Evidence review for active surveillance, radical prostatectomy or radical radiotherapy in people with localised prostate cancer NICE guideline NG13, Evidence reviews. 2019.
3. Merriel SWD, Hetherington L, Seggie A, et al, Best practice in active surveillance for men with prostate cancer: a Prostate Cancer UK consensus statement. BJUI 2019;124(1):47–54.
4. Hamdy FC, Donovan JL, Lane JA, et al, Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2023;388(17):1547–58.
5. Dearnaley D, Syndikus I, Mossop H, et al, Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP Trial. Lancet Oncol 2016;17(8):1047–60.
6. Montague DK. Artificial urinary sphincter: Long-term results and patient satisfaction. Advances in Urology 2012;2012:835290.