In the United Kingdom, almost 10,500 new cases of bladder cancer were identified in 2013, with over 5000 deaths in 2012 [1]. Seventy percent of new cases will be non-muscle invasive bladder cancer (NMIBC) at diagnosis and therefore will be managed in a bladder sparing manner with transurethral resection (TURBT), with or without intravesical therapy.
Initial management with TURBT can eradicate stage Ta or T1 transitional cell carcinoma (TCC) but risk of recurrence is high in these patients with a 50-70% risk of recurrence within five years and risk of progression to muscle invasive disease can be up to 50% [2]. Repeat treatments are costly and cause morbidity to patients so it is vital we use the most effective treatments available to mitigate this. Despite advances in all areas of medicine, the use of intravesical therapy is inconsistent worldwide and advances have been adopted slowly.
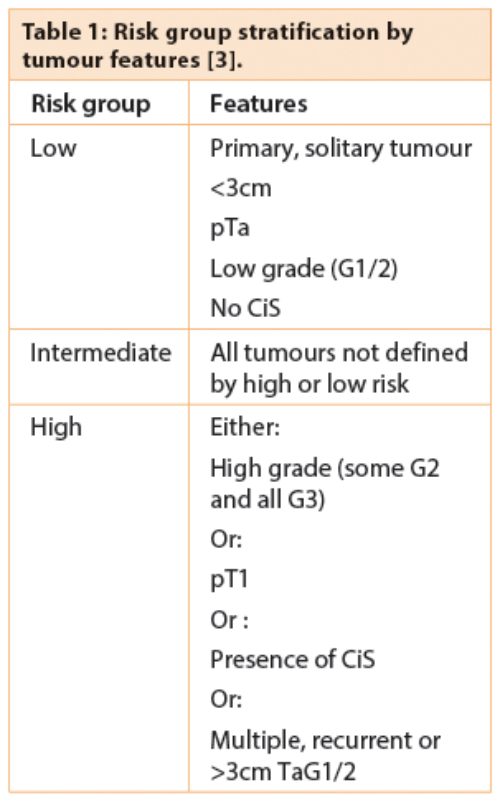
Use of intravesical therapy is guided by risk of progression and recurrence of individual tumours. The European Organization for Research and Treatment of Cancer (EORTC) Genito-Urinary Cancer Group (GUCG) has developed a scoring system and risk tables based on patients diagnosed with stage Ta or T1 tumours. A number of clinical and pathological factors are used to determine the score: number of tumours, tumour size, stage, grade, concurrent carcinoma in situ (CiS) and previous recurrence rate. These scores are then used to classify tumours as ‘low’, ‘intermediate’ or ‘high’ risk of progression or recurrence (Table 1), enabling treatment to be tailored to an individuals’ risk and potential benefit.
Chemotherapy
Intravesical chemotherapy can be used both as a single, immediate postoperative instillation, and as adjuvant chemotherapy. European Association of Urology (EAU) guidelines recommend an immediate post-TURBT instillation of chemotherapy to reduce recurrence [3]. This is proposed to destroy circulating cells and have an ablative effect (chemoresection) on residual tumour cells. The guidelines recommend this be done within two hours of TURBT, before the tumour resection site is covered by extracellular matrix.
Mitomycin C (MMC)
MMC is a low molecular weight alkylating agent that has low systemic absorption but good bladder wall penetration [4]. A 40mg dose is used in 40ml sterile water, administered intravesically unless there is a contraindication (suspected bladder wall perforation or muscle invasive disease).
Meta-analysis of randomised controlled trials has shown that instillation of intravesical chemotherapy immediately following TURBT prolongs the recurrence free interval by 38% compared to both no treatment and placebo [5]. This was statistically significant for MMC and epirubicin in tumours with low risk of recurrence and progression. This study does not convert the increase in recurrence free interval to an actual timescale of weeks, months or years but the follow-up period for the studies included in the meta-analysis ranges from 2 to 8.75 years. Current evidence supports administering the drug within two hours of TURBT and leaving it in the bladder for one hour for the greatest benefit [3]. There is no comparative data between different chemotherapy agents.
There is less robust guidance regarding the use of further adjuvant MMC. There is some evidence to support improved recurrence free survival in intermediate risk patients [3], particularly those with multiple tumours at presentation. Although there has been no consensus on the optimal duration of adjuvant intravesical chemotherapy, it is not recommended beyond one year [3].
Thiotepa
Another alkylating agent, Thiotepa, was first used in bladder cancer treatment in 1961 [4]. However, it has subsequently been shown to have an insignificant benefit and this, alongside the high risk of systemic absorption and serious side-effects, has meant it is no longer routinely used.
Epirubicin
Epirubicin is part of the anthracycline class of drugs and has a higher molecular weight resulting in reduced systemic absorption and toxicity [4]. As with MMC, there is evidence to support a single immediate post-resection dose of epirubicin in low-risk tumours with an increase in the recurrence free interval of 38% compared with both no treatment and placebo [5]. Although, as stated above, this does not convert into a specific time frame for disease free interval. Despite this proven efficacy, and its longer shelf life, epirubicin is not as commonly used as MMC. Unlike MMC, there has been no proven role for epirubicin beyond the initial postoperative instillation [4]. While it improves initial recurrence rates, it has not been proven beneficial in longer term use nor in prevention of progression.
Gemcitabine
Gemcitabine has proven activity against TCC and should have good cell penetration due to its lipid solubility [4]. Its high molecular weight also reduces systemic absorption. It has not yet been proven to be effective in preventing recurrence if used immediately post-resection but it has shown some promise as a salvage therapy in prevention of recurrence in patients who have developed recurrent disease despite treatment with MMC or Bacillus Calmette-Guerin (BCG) [6]. This study showed that the median time to recurrence was 15 months in these salvage patients but 37% of patients in this high-risk group remained recurrence free at 22 months [6]. This treatment seems most appropriate currently for patients who have failed conventional treatments but decline, or are not medically fit for, cystectomy.
Immunotherapy
Immunotherapy in the form of BCG was introduced into clinical use in urology in 1976. BCG is a live attenuated form of the slow-growing bovine tuberculosis bacterium and work in France developed a non-virulent strain that came into use as a vaccine in 1921 [7]. Ongoing work with BCG has resulted in multiple daughter strains becoming available for use. The most commonly used strain in the literature is Tice, with others including Connaught and Pasteur [2].
BCG
BCG is recommended for use in preventing disease recurrence and progression in intermediate and high-risk tumours [3]. However, it is not recommended in patients with low-risk tumours due to the already low risk of recurrence and progression [7] set against the side-effects of BCG treatment. The first reported schedule of BCG treatment is still used today in the form of induction BCG courses: one dose per week for six weeks [8]. BCG has been shown to be superior to TURBT alone, intravesical chemotherapy (immediate instillation) and maintenance MMC in prevention of recurrence [3]. Interestingly, there appears to be a reduction in efficacy in patients more than 70 years of age, but BCG was still more effective than comparable chemotherapy [3].
Meta-analysis of the published data has shown a significant reduction (27%) in progression to muscle invasive disease with maintenance BCG treatment use [2]. This group compared to control groups which included resection alone and also comparison to chemotherapy. Subset analyses of the benefit of BCG in Ta, T1 or CiS disease in these trials should be interpreted with caution due to size and study power but a few smaller trials have indicated that BCG does reduce progression in patients with CiS alone [3].
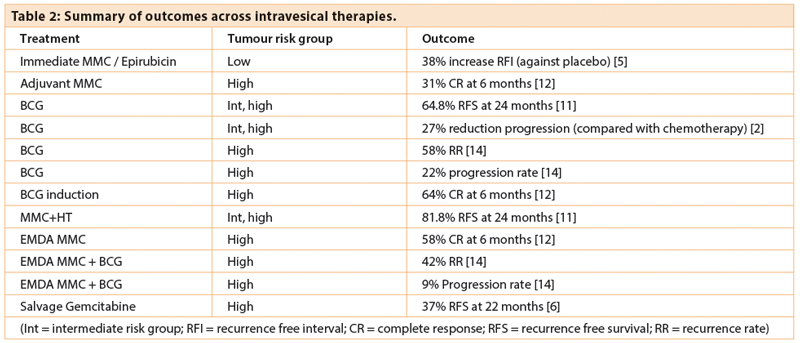
The exact length of maintenance treatment required has been a subject of much debate. An EORTC trial has shown that there is no additional benefit to having maintenance treatment beyond one year in intermediate-risk patients but in high-risk patients, three years of maintenance can reduce recurrences [9] (Table 2). There was no reduction in progression rates or death despite longer duration of maintenance in this high-risk group. It is recommended by EAU guidelines that only patients with high-risk disease receive greater than one year of maintenance BCG therapy [3].
In view of the different strains of BCG developed, a meta-analysis included subgroups of BCG strains and found no significant difference in treatment efficacy across strains used [2]. Patient intolerance of treatment schedules due to side effects, along with a recent shortage of BCG internationally, has led to lower dose regimens being widely used. The EORTC group showed no difference in toxicity between full dose and one-third dose treatments [9]. For intermediate-risk tumours, maintenance was only required for one year at full dose, with no added benefit over three years, but when receiving one-third of the dose, three years of treatment showed a greater reduction in recurrence [9]. They therefore recommend treatment with full dose BCG for a year in these patients despite other earlier studies claiming no difference in using one-third dose versus full dose.
BCG is well documented to cause more side- effects than intravesical therapy but it remains widely in use due to the proven efficacy in intermediate and high-risk disease. Patients who tolerate an induction schedule of BCG were also more likely to tolerate and complete maintenance treatment [3].
BCG failure
In some cases, patients develop high-grade recurrences during or after standard BCG therapy. In most cases, these patients should be offered radical cystectomy. However, in patients who are medically unfit or decline major surgery, ongoing BCG therapy is unlikely to work in the context of previous failure. Non-surgical options are still undergoing investigation and include chemotherapy, device assisted therapy and combination therapy [3].
Non-high grade recurrences and intolerance of BCG are other reasons that a treatment schedule may have to be altered. There is less evidence to guide treatment following BCG intolerance.
Device assisted therapy
Although there have been promising results, current guidelines suggest that delivery adaptations such as hyperthermia (HT) and electromotive drug administration (EMDA) are only used in experimental settings as outcomes are still not proven [3]. Much of the work with adjuncts to intravesical therapies has been with MMC.
Hyperthermia
HT has been utilised to improve efficacy of intravesical therapies and has been studied in bladder cancer since the early 1990s. HT alone can improve anti-tumour immunity and have direct cytotoxic effects, and it has been shown to increase cytotoxicity and improve absorption of MMC [10]. There have been a number of delivery systems developed; commonly this is delivered locally via a Foley catheter at temperatures of 41-45oC, but there have been some external delivery systems utilising both chemotherapy and radiotherapy [10]. Local delivery systems have been CE approved for use in Europe for the treatment of NMIBC.
Microwave systems, such as the approved Synergo® system, utilise an intravesical radiofrequency energy applicator on a Foley catheter and these systems have been proven to be effective above 42oC. With the microwave systems, cooled chemotherapy solution is instilled then heated intravesically via direct irradiation from the energy applicator on the tip of a more rigid 20F catheter. This can reduce thermal injury to the urethra. Most of the currently available literature utilises microwave systems.
"Repeat treatments are costly and cause morbidity to patients so it is vital we use the most effective treatments available to mitigate this."
The Elmedical conductive-heat based system for bladder wall thermo-chemotherapy (UniThermia) provides thermo-chemotherapy instillation of a heated 40-80mg Mitomycin C solution (in water) at a uniform high temperature 44-44.5oC over the entire bladder lumen by means of fast circulation through the bladder via a more flexible 18F catheter. With this system, the chemotherapy is heated outside of the patient prior to delivery to the bladder which allows a more consistent temperature and can reduce burns [10].
Another unit currently being utilised in trials in the United Kingdom is the Combat BRS system. This works in a similar fashion to the Elmedical device, in that the chemotherapy solution is heated prior to instillation via a standard three way catheter. 40mg of Mitomycin C in solution is heated via an aluminium heat exchanger to 43oC. There is promising early evidence with this system but larger trials are underway.
A randomised controlled trial has shown a statistically significant improvement in recurrence free survival over 24 months comparing intravesical chemohyperthermia with MMC versus BCG (using the Synergo system) [11]. Although this study was under powered, the results show 81.8% recurrence free survival at 24 months in the HT group compared with 64.8% in the BCG group. Progression rates were comparable. They have concluded that HT is a safe and effective treatment option in patients with intermediate and high-risk NMIBC [11]. The safety, efficacy and side-effect profile of these treatments needs further evaluation if HT with chemotherapy is to replace BCG, a proven efficacious treatment but limited by side-effects. Once stand-alone trials with individual devices provide significant results, there will then need to be further studies comparing different systems, such as microwave (Synergo) versus conductive heat systems (UniThermia / Combat BRS).
Electromotive drug administration
Electrokinetic forces can improve drug delivery across and into biological membranes. MMC is a polar, non-ionised molecule in typical urine which can be delivered by electro-osmosis [12]. EMDA uses an electric current to enhance trans-epithelial drug penetration. A battery-powered generator delivers an electric current which passes between two electrodes: one in the bladder (on a catheter), and one on the skin of the lower abdomen [13]. The use of EMDA with MMC was first looked into in the 1990s although EMDA as a concept has been around at least a decade longer.
A randomised study has shown a statistically significant difference in complete response of high risk T1 and Tis tumours with electromotive MMC (58%) compared with passive MMC (31%) [12]. This is comparable to the response rate of similar tumours to BCG (64%). The same study showed that local and systemic side-effects were more prominent with BCG than either delivery of MMC and thus could be considered a better tolerated alternative for certain patients. Subsequently, the same group has compared sequential BCG and EMDA MMC with BCG alone with results showing significantly improved disease free survival (69 vs. 21 months) and a reduction in both recurrence rates (42% vs. 58%) and progression rates (9% vs. 22%) [14].
Conclusion
Although current treatment regimens for NMIBC have been shown to be effective (Figure 2), further clarification of the optimal agent and means of delivery is needed to improve efficacy. A uniform approach of ‘one size fits all’ in intravesical therapy is inappropriate, so regimes should be tailored to individual risk and refined to reduce recurrence rates and progression in this common disease.
References
1. Cancer Research UK.
http://www.cancerresearchuk.org/health-professional/
cancer-statistics/statistics-by-cancer-type/
bladder-cancer/incidence#heading-Three
Last accessed May 2016.
2. Sylvester RJ, van der Meijden APM, Lamm DL. Intravesical Bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. Journal of Urology 2002;168(5):1964-70.
3. Babjuk M, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. European Urology 2013;64(4):639-53.
4. Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer – indications and limitations. BJU International 2012;110(Suppl 4):12-21.
5. Perlis N, Zlotta AR, Beyene J, et al. Immediate Post-Transurethral Resection of Bladder Tumor Intravesical Chemotherapy Prevents Non-Muscle-invasive Bladder Cancer Recurrences: An Updated Meta-analysis on 2548 Patients and Quality-of Evidence Review. European Urology 2013;64(3):421-30.
6. Cockerill PA, Knoedler JJ, Frank I, et al. Intravesical gemcitabine in combination with mictomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU International 2016;117(3):456-62.
7. Kamat AM, Flaig TW, Grossman B, et al. Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nature Reviews Urology 2015;12(4):225-35.
8. Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. Journal of Urology 1976;116(2):180-3.
9. Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU Cancers Group Randomized Study of Maintenance Bacillus Calmette-Guerin in Intermediate- and High-risk Ta, T1 Papillary Carcinoma of the Urinary Bladder: One-third Dose Versus Full Dose and 1 Year Versus 3 Years of Maintenance. European Urology 2013;63(3):462-72.
10. Owusu RA, Abern MR, Inman BA. Hyperthermia as Adjunct to Intravesical Chemotherapy for Bladder Cancer. Biomed Research International 2013; Epub ahead of print.
11. Arends TJH, Nativ O, Maffezzini M, et al. Results of a Randomised Controlled Trial Comparing Intravesical Chemohyperthermia with Mitomycin C Versus Bacillus Calmette-Guerin for Adjuvant Treatment of Patients with Intermediate- and High-risk Non-Muscle-invasive Bladder Cancer. European Urology 2016; Epub ahead of print.
12. Di Stasi SM, Giannantoni A, Stephen RL, et al. Intravesical electromotive Mitomycin C versus passive transport Mitomycin C for high risk superficial bladder cancer: A prospective randomized study. Journal of Urology 2003;170(3):777-82.
13. Slater SE, Patel P, Viney R, et al. The effects and effectiveness of electromotive drug administration and chemohyperthermia for treating non-muscle invasive bladder cancer. Annals of the Royal College of Surgeons of England 2014;96(6):415-19.
14. Di Stasi SM, Giannantoni A, Giurioli A, et al. Sequential BCG and electromotive mitomycin versus BCG alone for high-risk superficial bladder cancer: a randomised controlled trial. Lancet Oncology 2006;7(1):43-51.
Declaration of Competing Interests: None declared.