What are posterior urethral valves?
Posterior urethral valve (PUV) is the most common cause of congenital bladder outlet obstruction (BOO) and renal failure in male children. They were first described by the Italian anatomist Giovanni Battista Morgagni back in the 1700s [1].
PUV represents an overgrowth of normally present anatomical folds in the urethra. The embryological origin of these folds remains unclear. Their location in the posterior urethra, which is an area of complex embryology, led to the development of different theories. On histology, Lowlsey found connective tissue similar in structure to the sheath enclosing the ejaculatory ducts concluding that PUV could possibly originate from this sheath. Hugh Hampton Young in 1919 was the first to describe the pathology endoscopically, classifying valves according to their configuration into three types [1]; this is demonstrated in Figure 1.

Figure 1: Types I–III as described by Young. Type I is the most common (80–85%), followed by type III (10–15%), type II is rarely identified. Posterior urethral valves (arrow), bladder neck (*), verumontanum (v), external urethral sphincter (S).
How common are PUVs?
Based on recent national audit data, the calculated incidence of PUV in the UK is 1/3800 live births per annum. Of these cases, only 35% were diagnosed antenatally, 42% during infancy, and 23% after the age of one [2]. Nevertheless, a non-negligible percentage of PUV patients present in late childhood, but the actual incidence is probably under-estimated [3].
What is the pathophysiology of PUVs?
At early stage, BOO caused by PUV is compensated by detrusor hypertrophy and high voiding pressures, which can be followed by decompensation and detrusor failure in some untreated cases. These changes can cause progressive upper tract deterioration and renal failure which would be proportionate to severity and duration of the obstruction. Age at presentation has been found to be a surrogate marker of severity, and a predictor of upper tract deterioration and progression to end-stage kidney disease [3].
What is the index case?
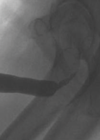
The index case is typically identified on antenatal ultrasound scan, or early postnatally. Ultrasound shows bilateral hydroureteronephrosis, distended and thick-walled bladder and dilated posterior urethra with the characteristic keyhole sign. These findings are generally confirmed by a micturating cystourethrogram (MCUG) performed after birth. Other possible signs on MCUG are bladder trabeculations, dilated posterior urethra, hypertrophied bladder neck (spinning top sign) with or without vesico-ureteric reflux (Figure 2). This index case represents the severest form of the disease, which most clinicians are familiar with. However, milder cases that present during late childhood, may not demonstrate all these cardinal signs [4].


Figure 2: Top: ultrasound scan images showing hydronephrosis, and key-hole sign.
Bottom: MCUG showing dilated posterior urethra with transition point at level of PUV, and high grade reflux.
Why do some PUVs present late?
As with all congenital anomalies, PUVs represent a broad spectrum; one end represents the index case with obvious structural and functional abnormalities, whilst the opposite end of the spectrum is very mild and close to normal. Those at the mildest end of the spectrum typically present after the age of potty-training when urinary symptoms arise. As already stated, age at presentation has been shown to be a defining feature of severity in various studies [3].
What is the age cut-off for late-presenting PUV?
There is no consensus on the ideal cut-off for the definition of late-presenting PUV. There are a few classifications; the most commonly used is antenatal versus postnatal, with postnatal further divided into infantile and late presentation. It is believed that PUV presenting in late childhood (after the age of five) represents the mildest end of the spectrum and the most challenging to diagnose, given the subtle presentation and clinical picture [3].
How do late-presenting PUVs present?
They present with lower urinary tract symptoms (LUTS) mostly storage (65%) including frequency, urgency, urge incontinence, and nocturnal enuresis. Depending on degree / duration of obstruction and compensatory mechanisms, some patients would also report voiding symptoms such as weak flow, need to strain, and urinary retention (15%). Other common symptoms are recurrent infections and haematuria in 20% [3].
How do you diagnose late-presenting PUVs?
Given the mild nature of the condition, they represent a significant diagnostic challenge. Moreover, LUTS are quite common in children and teasing out cases that could have an underlying urethral pathology requires a high level of suspicion. Here we report our recommendations:
1. Identify suspicious cases early:
a. Get uroflowmetry in boys with LUTS at first consultation
Uroflowmetry is an essential screening tool for children with refractory LUTS. It should be interpreted with consideration of patient age and voided volume using a validated nomogram (e.g., Tzu Chi nomogram). Those with a maximum flow rate (Qmax) below 5th centile for age, abnormal plateau pattern, and / or high post-void residual urine are at highest risk of having urethral obstruction. Although, one should bear in mind that normal flow can still be sustained at the cost of high detrusor pressure. In those cases, lack of response to conservative measures, also known as urotherapy, should raise suspicion [5].
b. Get an ultrasound scan in those with abnormal flow and / or non-respondents to urotherapy
Findings such as hydro-uretero-nephrosis, thickened bladder wall, high post-void residuals, and / or dilated posterior urethra are suggestive of PUV. However, a significant percentage of late presenters may have normal ultrasound scans at presentation [4].
2. Confirmation of diagnosis:
There is no agreed diagnostic algorithm for children with refractory LUTS and suspected urethral obstruction [4]. Options for investigations include:
a. MCUG
Positive findings are similar to those in the index case. However, MCUG requires urethral catheterisation which is a significant undertaking for a child or teenager, and also involves radiation exposure. In addition, it is subjective and can be inconclusive in 40% of cases. In our practice, we perform MCUG only as part of a video urodynamic study (VUD), this allows us to maximise the information obtained [6].
b. Pressure flow study (PFS) and VUD
PFS is the gold-standard diagnostic tool for BOO, which is characterised by high detrusor voiding pressure (Pdet) and associated low flow rate. The Abrams-Griffiths nomogram has been widely used in adults to diagnose BOO. BOO index (BOOI) is calculated as (Pdet-Qmax – [2 X Qmax]); if the value is >40 that is considered obstructed, <20 is not obstructed, and 20–40 is equivocal [7]. This index has not been validated in children yet, but a recent study showed correlation of male adult BOOI with bladder dysfunction and presence of residual obstruction in children post-PUV ablation, which shows a promise for wider application in the paediatric population [8]. A limitation of PFS in this age group is insertion of a urethral line which would typically require sedation. Also, urine flow can be limited by it, given the small urethral calibre in children. An alternative is to insert suprapubic lines. We prefer VUD as it secures a more definitive diagnosis. VUD provides both structural and functional information of MCUG and PFS as discussed above. It can also show reflux which takes off voiding pressure leading to underestimation of BOO.

Figure 3: Endoscopic view of PUV: flaps (arrows) that arise from base of verumontanum (star).
c. Rigid cystoscopy:
On rigid cystoscopy, signs to look for include: i) dilated posterior urethra, ii) high bladder neck, and iii) trabeculated bladder wall. The valves appear endoscopically as flaps extending distally from the base of the verumontanum to each side wall of the prostatic urethra (Figure 3). Our recommendation is to assess the presence of PUV carefully both with anterograde and retrograde movement of the cystoscope. The degree of bladder filling, the pressure of irrigation-flow, and the experience of the surgeon can result in significant inter-observer variability, which is more profound in subtle cases. A study showed lack of consensus even among experts in detecting PUV on endoscopic views [9].
3. Further recommendation:
In our practice, we perform cystoscopy on those with suspected urethral obstruction. If PUV is identified, we proceed to endoscopic valve ablation (EVA). In case of inconclusive findings, we insert suprapubic lines and perform PFS combined with MCUG as part of VUD, later the same day or the following day. Those with suspected BOO on VUD would be taken back to theatres for EVA on a later date.
4. Future prospects:
Artificial intelligence (AI) might have a greater role in identifying those at higher risk of having PUV, possibly reducing the number of cases going through unnecessary investigations and allowing prompt treatment for those at highest risk. Deep artificial neural network could predict 92.7% of PUV cases in three to seventeen-year-old boys with refractory LUTS [10].
What is the management of late-PUVs?
The main step is endoscopic ablation using cold-knife incisions at 5 and 7 o’clock. Another incision at 12 o’clock can be required in severe cases. Thermal ablation is not favoured by the author as it could be associated with a higher risk of complications such as urethral stricture.
"Cases with high suspicion should be referred early to a specialist centre for a definitive diagnosis"
The outcome is generally good. However, most studies relied on subjective report of symptomatic improvement with some 70% complete / significant response, 10% partial improvement requiring additional treatment, and 20% persistent symptoms. In those with residual symptoms despite ablation, residual obstruction should be ruled out which can be secondary to residual valves (incomplete resection), bladder neck obstruction dysfunctional voiding and / or urethral stricture. In our protocol we perform a check cystoscopy and video urodynamic study generally three months after ablation [3].
How to follow-up these children?
Although effective de-obstruction is the most important first step in management, optimisation of detrusor function is probably the determinant step for long-term outcome. Untreated bladder dysfunction can result in upper tract deterioration. Studies showed up to 60% of late-presenting PUV had bladder dysfunction which required pharmacological treatment, with 17% developing chronic kidney disease and 6% ending in end-stage renal disease [3].
In our practice, we arrange annual review for these children until they are transitioned to adult practice, assessing their: (i) symptoms, (ii) uroflowmetry, (iii) ultrasound scan, (iv) renal profile, and (v) urine microscopy for proteins. Interval VUD and DMSA-scan to check for renal scarring are also performed and repeated prior to transition.
TAKE HOME MESSAGES
-
Late presenting PUV is a rare and complex condition.
-
Urethral obstruction should be suspected in boys with refractory LUTS.
-
Boys with abnormal uroflowmetry, ultrasound scan findings, and/ or refractory symptoms such as recurrent UTIs, and haematuria should be referred early to specialist care.
-
Late-presenting PUV patients need long-term follow-up due to the risk of bladder dysfunction and upper tract deterioration.
References
1. Krishnan A, De-Souza A, Konijeti R, et al. The anatomy and embryology of posterior urethral valves. J Urol 2006;175(4):1214–20.
2. Brownlee E, Wragg R, Robb A, et al. Current epidemiology and antenatal presentation of posterior urethral valves: Outcome of BAPS CASS National Audit. J Pediatr Surg 2019;54(2):318–21.
3. Gabrielson AT, Galansky LB, Florissi I, et al. Infantile versus childhood posterior urethral valve diagnosis: management patterns and clinical outcomes at opposite ends of the spectrum. J Pediatr Urol 2023;19(5):638.e1–8.
4. Hennus P, de Kort L, Bosch J, et al. A systematic review on the accuracy of diagnostic procedures for infravesical obstruction in boys. PLoS One 2014;9(2):e85474.
5. Winck-Flyvholm L, Pedersen KD, Hildorf S, et al. Evaluation of boys with daytime incontinence by combined cystourethroscopy, voiding cystourethrography and urodynamics. Scand J Urol 2021;55(3):249–56.
6. Özen MA, Taşdemir M, Gökhan Gündoğdu, et al. Does voiding cystourethrogram exclude posterior urethral valves in late presenting cases? Eur J Pediatr Surg 2019;29(1):85–9.
7. Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995;13:34–9.
8. Vaze PG, Saha S, Sinha R, et al. Urodynamics in posterior urethral valve: pursuit of prognostication or optimisation. J Pediatr Urol 2021;17(1):111.e1–8.
9. De Jong T, Radmayr C, Dik P, et al. Posterior urethral valves: search for a diagnostic reference standard. Urology 2008;72(5):1022–5.
10. Abdovic S, Cuk M, Cekada N, et al. Predicting posterior urethral obstruction in boys with lower urinary tract symptoms using deep artificial neural network. World J Urol 2019;37(9):1973–9.
Declaration of competing interests: None declared.