Urethral stricture is the most common cause of lower urinary tract obstruction in men aged between 20 and 40, carrying an estimated overall prevalence of 0.5% in the UK [1] and results in around 17,000 hospital admissions annually [2]. Endoscopic management, by urethral dilatation or optical urethrotomy, has traditionally been the mainstay of surgical treatment, however high recurrence and poor long-term success rates have led to the development of novel techniques.
Reconstructive surgery, namely urethroplasty, is an increasingly common option in the surgical management of both primary and recurrent urethral stricture, and produces encouraging long-term results. This article will discuss the basis for urethroplasty, the techniques involved and the current evidence-base behind the trend towards this treatment, particularly in the context of stricture disease.
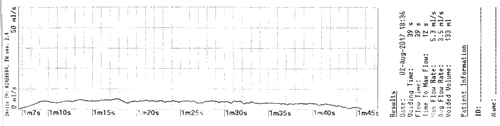
Figure 1. Example of a uroflowmetry trace in a patient with urethral stricture.
Aetiology
The male urethra can be divided into anterior and posterior parts, distal and proximal to the external urinary sphincter respectively. The anterior urethra is composed of the bulbar and penile urethrae and is more susceptible to stricture disease. The posterior urethra is composed of the membranous and prostatic urethrae.
Urethral stricture is defined as an abnormal narrowing of the urethra, thought to be caused by varying degrees of spongiofibrosis – scarring of the corpus spongiosum surrounding the urethra. Anatomical location is important – the majority of strictures affect the anterior urethra, with 50% confined to the bulbar urethra, 20% penile and 30% the navicular fossa [1]. Posterior urethral strictures are rare, the majority resulting from pelvic trauma or following prostatic radiotherapy. Due to the rarity and differing techniques used in the management of posterior urethral strictures and posterior urethroplasty, it will be discussed in a separate article.
Aetiology is broadly categorised into traumatic, infective and inflammatory. An estimated 45% of strictures are caused by iatrogenic injury following transurethral instrumentation or traumatic catheterisation [1], and it has been reported that up to 5% of patients will develop a urethral stricture following transurethral resection of the prostate (TURP) [1,3]. Untreated sexually-transmitted infections, most commonly gonorrhoea, result in chronic bacterial urethritis and cause up to 20% of cases of urethral stricture. Less common causes include congenital hypospadias, chronic inflammatory dermatological conditions – for example balanitis xerotica obliterans / lichen sclerosus [4] – and external-beam radiotherapy or brachytherapy in the treatment of prostate cancer. Around 30% of urethral strictures are considered idiopathic, however it is hypothesised that many of these cases will in fact have resulted from forgotten historical minor trauma [5].
Diagnosis
Presenting symptoms tend to be related to chronic voiding dysfunction, with most patients complaining of a poor urinary stream and a feeling of incomplete bladder emptying, or with symptoms of recurrent urinary tract infections or sexual dysfunction. Less commonly, patients present with symptoms of prostatitis, epididymitis or acute urinary retention requiring emergency transurethral or suprapubic catheterisation [6]. Non-invasive investigative methods such as uroflowmetry, the results of which produces a pathognomonic curve with a prolonged voiding time and low-level flow, and ultrasound post-void residual measurements will demonstrate reduced bladder emptying.
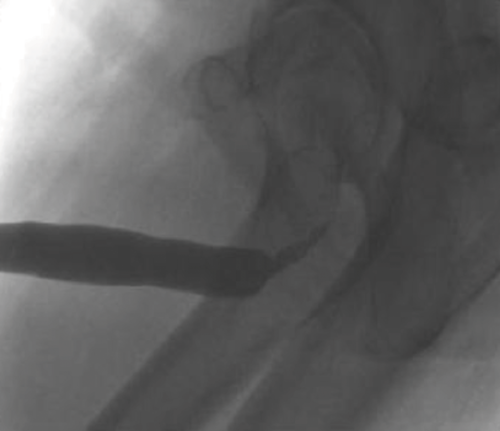
Endoscopic visualisation via cystourethroscopy can identify the location of the stricture but provides minimal information on stricture length. Ultrasonography is useful in demonstrating depth of spongiofibrosis, whilst contrast studies such as retrograde urethrography and voiding cystourethrography accurately demonstrate the anatomical location and length of the stricture, which guides further treatment. An example of a urethrogram can be seen in Figure 2.
Figure 2. Ascending urethrogram demonstrating bulbar urethral stricture.
Current guidelines
There are few large-scale studies relating to the management of urethral stricture, and to date neither the British Association of Urological Surgeons (BAUS) nor the European Association of Urology (EAU) have produced official guidelines. The American Urological Association (AUA) published a guideline in 2016 and Société Internationale d’Urologie (SIU) in 2010 [7], both of which advocate urethroplasty as the definitive treatment of recurrent urethral stricture disease and also as a primary treatment in specific circumstances, for example in penile urethral strictures and hypospadias. The AUA advises urethroplasty in all cases of recurrence [7]; in contrast to the SIU which does advocate endoscopic treatment via dilatation or urethrotomy in specific cases of recurrent stricture [7].
The ‘OPEN’ trial [8] is a randomised, pragmatic, multicentre trial, currently taking place in the UK, which aims to compare results of open urethroplasty with repeat endoscopic urethrotomy in the management of recurrent bulbar urethral stricture. It will follow-up patients over a two-year period to compare a range of factors from symptom improvement and quality of life to overall economic cost of each treatment. Follow-up was due to be completed in December 2017, with the results of this trial of great interest to specialists.
Management
Traditionally, urethral stricture was managed endoscopically, most commonly by urethrotomy, with the stricture scar incised to widen the urethral lumen and healing by secondary intention, however reported recurrence rates are as high as 60% in the literature [9,10]. One prospective randomised trial found that, in terms of recurrence rate, urethrotomy is no more effective than historical urethral bougienage methods [11]. Poor long-term outcomes from these endoscopic methods have presented an opportunity for open reconstruction – urethroplasty – to develop.
Urethroplasty surgery can be described as open reconstruction or repair of the urethra, and is performed most commonly due to urethral strictures of varying aetiology, but also in trauma and congenital conditions such as hypospadias. Although not frequently first-line due to increased risks of open surgery, it is regarded as the gold standard in stricture management, often offering superior outcomes and lower stricture recurrence rates than the aforementioned urethral dilatation and urethrotomy [12-14]. Due to this, there is merit in early consideration in suitable patients. In the period 2014-2015, 763 urethroplasties were performed in the UK, compared with 4583 optical urethrotomies and 8273 endoscopic urethral dilatations over the same time period [15].
Of several variants of the technique, the most common types of anterior urethroplasty practised In the UK are anastomotic and augmentation urethroplasty, the latter using either buccal or lingual mucosal grafts. As a general rule, anastomotic urethroplasty is favoured in shorter-segment excisional or traumatic repairs, while augmentation is utilised in the management of longer (>1.5-2cm), recurrent or complex strictures [12,13].
However, in practice, different techniques may be utilised depending on size, location, number and nature of stricture(s), availability of autograft tissue, along with patient fitness and desires.
Anastomotic urethroplasty
First described by Young and Marion at the beginning of the 20th century, anastomotic urethroplasty involves the direct, end-to-end joining of the urethra following excision of the affected tissue. As aforementioned, it is generally accepted in the literature that it is favoured in transecting trauma and anterior strictures <1.5cm in length [12,13].
After effective general anaesthesia, positioning in the Lloyd-Davies position and aseptic preparation / draping of the patient, careful dissection of the dermal and sub-dermal layers of the ventral aspect of the penis or perineum is performed. Following exposure of the corpus cavernosum, spongiosum and ventral urethra, positioning or ‘stay’ sutures are inserted and the affected length of urethra is marked. Gentle separation of the urethra from the cavernosum is performed, and a retractor is placed dorsal to the urethra to protect other structures before resection of the affected segment. Following this, a catheter is passed through the urethra to allow for approximation of the patent ends and the two ends of the transected urethra are spatulated in preparation for anastomosis, from dorsal to ventral. A Foley catheter is subsequently inserted to allow passage of urine while protecting the anastomosis, although occasionally a supra-pubic catheter is preferred.
Defined as the absence of complications or symptoms of recurrent stricture, ‘success’ rates for anastomotic urethroplasty vary in the literature, with rates as high as 95% [16] to 98.8% [12] reported.
Augmentation / substitution urethroplasty
This technique utilises autograft tissue to form a length of urethra in either a one or two-stage technique, depending primarily on the location and length of the stricture. Differing types of autograft tissues have been described, with oral (buccal and / or lingual) mucosal grafts (OMG) being widely adopted, but reasonable success has also been achieved with the use of scrotal skin [17], bladder mucosa and colonic mucosa [18]. Tissue engineering of grafts is also an emerging option in problematic cases, but has shown mixed results [19].
First described by Humby in 1941 [20], OMG urethroplasty is now widely used in contemporary practice. OMG is an ideal urethral substitute due to tissue similarities, versatility for use in different urethral locations, compatibility in a wet environment and ease of harvest [21]. Techniques involved in preparation, approach and stricture excision are similar to those used in anastomotic urethroplasty, with the addition of a simultaneous harvesting of an OMG by a reconstructive urologist either as a one or two-team approach. The OMG is then prepared to fit the size of stricturotomy and often fenestrated before onlay or tubulisation and suturing onto the urethral defect. As previously mentioned, this can form part of a one or two-stage technique. In a one-stage technique, the OMG can be used to augment an anastomotic repair or a short segment excision, whereas in a two-stage technique, a ‘roof strip’ may be formed and then followed by a second-stage tubulisation of the urethra at a later date. This is more common in the management of longer strictures or defects. A Foley catheter is again inserted to protect the reconstructed urethra.
OMG donor site management remains an area of debate. Once harvested, the defect can either be closed primarily with absorbable sutures or left to heal by secondary intention. Despite differences in opinion, it has been shown that OMG donor sites heal successfully regardless of technique used [21], but that an improvement in postoperative pain and oral intake can be seen with primary closure [22].
“Urethroplasty surgery can be described as open reconstruction or repair of the urethra, and is performed most commonly due to urethral strictures of varying aetiology, but also in trauma and congenital conditions.”
Reported success rates in OMG urethroplasty vary depending on several factors, such as one vs. two-stage technique, positioning of graft (dorsal vs. ventral) and autograft tissue type. With success rates varying from 65.8% [23] to 87% [24] for dorsal onlay grafting, it is clear that the success rates are somewhat less than in anastomotic urethroplasty. Barbagli et al. [25] analysed a cohort of 375 patients and found a success rate of 90.9% for anastomotic urethroplasty, 60% for augmented anastomotic repair and 80% for OMG onlay techniques. However, it may be the case that these techniques are not directly comparable due to the influence of other variables such as increased stricture length and location in augmented / substitution urethroplasties.
Postoperative management
Aside from routine postoperative management of any complications, wound care and analgesia, management of all types of urethroplasty patients involves monitoring of the urethral catheter and subsequent interval peri-catheter urethrogram (PUG) to confirm healing and facilitate catheter removal. Although the optimal interval at which to perform PUGs and remove catheters is still debated, a review of several papers examining the time to catheter removal reported a range of 7-14 days in anastomotic and 7-28 days in augmentation / substitution urethroplasties [26,27].
Urodynamics is another widely-adopted technique of assessing potential improvement in urinary flow postoperatively. This includes uroflowmetry and post void residual (PVR), which are useful measurements in monitoring progress.
Management of co-morbidities such as diabetes and cardiovascular disease is important and BAUS emphasise this in addition to lifestyle advice, such as smoking cessation and gradual return to driving, as vital aspects of postoperative management [14].
Complications
The more common postoperative complications of urethroplasty include swelling and bruising around the wound, numbness or discomfort around the buccal mucosal graft donor site or spraying of urine (all up to 50% of patients). Less common complications include erectile dysfunction, wound infection (up to 10%), urinary fistula and anastomotic leak (around 2%).
As previously mentioned, stricture recurrence is another problematic complication, with rates of up to 14% for anastomotic and 42% for augmentation / substitution urethroplasty reported in the literature, after long-term (10+ years) follow-up [28]. However, through comparison with stricture recurrence rates in other treatments (up to 60% in urethrotomy [9,10]), it is apparent that urethroplasty offers superior outcomes in terms of stricture recurrence.
Conclusion
The increasing trend towards the utilisation of urethroplasty and evidence presented in the literature would suggest that it is a safe and effective means of management of anterior urethral stricture disease. This is evidenced by high success rates, particularly in anastomotic urethroplasty, and low stricture recurrence rates, when compared with other treatment modalities.
There are, however, no widely-adopted or published guidelines on urethroplasty type or technique and it is clear that further study, perhaps in the form of prospective RCTs, is required to help guide their development. It is important that management decisions are taken on an individual basis as every case presents unique challenges in the form of location and length of stricture, along with other patient factors.
References
1. Open urethroplasty versus endoscopic urethrotomy - clarifying the management of men with recurrent urethral stricture (the OPEN trial): study protocol for a randomised controlled trial.
https://www.opentrial.co.uk.
Accessed 02/10/2017.
2. Fenton AS, Morey AF, Aviles R, Garcia CR. Anterior urethral strictures: etiology and characteristics. Urology 2005;65:1055-8.
3. Das S, Tunuguntla HS. Balanitis xerotica obliterans – a review. World J Urol 2000;18:382-7.
4. Santucci RA, McAninch JW. Urethral reconstruction of strictures resulting from treatment of benign prostatic hypertrophy and prostate cancer. Urol Clin North Am 2002;29:417-27.
5. Tonkin JB, Jordan GH. Management of distal anterior urethral strictures. Nat Rev Urol 2009;6(10):533-8.
6. Bayne D, Gaither T, Awad M, et al. Guidelines of guidelines: a review of urethral stricture evaluation, management, and follow-up. Translational Andrology and Urology 2017;6(2):288-94.
7. Stephenson R, Carnell S, Johnson N, et al. Open urethroplasty versus endoscopic urethrotomy - clarifying the management of men with recurrent urethral stricture (the OPEN trial): study protocol for a randomised controlled trial. Trials 2015;16(1):600.
8. Pansadoro V, Emiliozzi P. Internal urethrotomy in the management of anterior urethral strictures: long-term follow-up. J Urol 1996;156:73-5.
9. Verges J, Desgrez JP, Claude JM, Cabane H. Internal urethrotomy. Resection of urethral stricture (over 5 years follow-up). Ann Urol 1990;24:73-5.
10. Steenkamp JW, Heyns CF, de Kock ML. Internal urethrotomy versus dilation as treatment for male urethral strictures: a prospective, randomized comparison. J Urol 1997;157:98-101.
11. Eltahawy EA, Virasoro R, Schlossberg SM, et al. Long-term follow up for excision and primary anastomosis for anterior urethral strictures. J Urol 2007;177:1803-6.
12. Tinaut-Ranera J, Arrabal-Polo MA, Merino-Salas S, et al. Outcome of urethral strictures treated by endoscopic urethrotomy and urethroplasty. Can Urol Assoc J 2014;8(1-2):E16-E19.
13. www.baus.org.uk/_userfiles/pages/files/
Patients/Leaflets/Bulbar%20urethroplasty.pdf.
Accessed 02/10/2017.
14. http://content.digital.nhs.uk/searchcatalogue?
productid=19420&q=title%3a%22Hospital+
Episode+Statistics%2c+Admitted+patient+care+-+
England%22&sort=Relevance&size=10&page=1#top.
Accessed 02/10/2017.
15. Santucci RA, Mario LA, McAninch JW. Anastomotic urethroplasty for bulbar urethral stricture: analysis of 168 patients. J Urol 2002;167(4):1715-19.
16. Jordan GH. Scrotal and perineal flaps for anterior urethral reconstruction. Urol Clin North Am 2002;29:411-16.
17. Xu YM, Qiao Y, Sa YL, et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol 2009;182(3):1040-3.
18. El-Kassaby A, Aboushwareb T, Atala A. Randomized comparative study between buccal mucosal and acellular bladder matrix grafts in complex anterior urethral strictures. J Urol 2008;179(4):1432-6.
19. Humby G. A one-stage operation for hyposadius repair. Br J Surg 1941;29:84–92.
20. Zimmerman WB, Santucci RA. Buccal mucosa urethroplasty for adult urethral strictures. Indian J Urol 2011;27(3):364-70.
21. Wong E, Fernando A, Alhasso A, Stewart L. Does closure of the buccal mucosal graft bed matter? Results from a randomized controlled trial. Urology 2014;84(5):1223-7.
22. Barbagli G, Morgia G, Lazzeri M. Dorsal onlay skin graft bulbar urethroplasty: long-term follow-up. Eur Urol 2008;53:628-33.
23. Pisapati VL, Paturi S, Bethu S, et al. Dorsal buccal mucosal graft urethroplasty for anterior urethral stricture by Asopa technique. Eur Urol 2009;56:201-5.
24. Barbagli G, Guazzoni G, Lazzeri M. One-stage bulbar urethroplasty: retrospective analysis of the results in 375 patients. Eur Urol 2008;53:828-33.
25. Al-Quadah HS, Cavalcanti AG, Santucci RA. Early catheter removal after anterior anastomotic (3 days) and ventral buccal mucosal onlay (7 days) urethroplasty. Int Braz J Urol 2005;31(5):459-64.
26. Solanki S, Hussain S, Sharma DB, et al. Evaluation of healing at urethral anastomotic site by pericatheter retrograde urethrogram in patients with urethral stricture. Urol Ann 2015;6(4):325-7.
27. Andrich DE, Dunglison N, Greenwell TJ, Mundy AR. The long-term results of urethroplasty. J Urol 2003;170:90-2.
Take home message
-
Urethral stricture carries an overall prevalence of 0.5% in the UK and is the most common cause of lower urinary tract obstruction in young men.
-
Common causes include: trauma, inflammatory (such as balanitis xerotica obliterans) and infective (such as sexually-transmitted infections).
-
Although traditionally treated with urethral dilatation or urethrotomy, urethroplasty is becoming increasingly popular as a surgical management method, A superior outcomes, with curative intent.
-
There are two main types of urethroplasty: anastomotic and augmentation / aubstitution. These are utilised in different presentations, mainly dependent on stricture length and location.
-
Oral mucosa graft (buccal and / or lingual) urethroplasty is increasingly used due to autograft tissue suitability and favourable outcomes.
-
However, there are a lack of established guidelines in technique selection.
Declaration of competing interests: None declared.