Catheterisation is a common medical procedure in which a catheter (a hollow flexible tube) is inserted into the bladder in order to facilitate the drainage of urine. Catheters are usually passed into the bladder via the urethra, either to be left indwelling or to be periodically inserted and removed as required throughout the day (intermittent catheterisation).
Less commonly, suprapubic catheters may be used following the surgical creation of a connection from the skin directly into the bladder above the pubic bone, through which a catheter may be passed and exchanged. Standard ‘Foley’ catheters are typically made of latex and are available in a variety of lengths and diameters depending on the gender and individual anatomy of the patient, though many permutations are available.
Catheterisation may be performed for a variety of reasons including urine-output monitoring in critical illness, intraoperatively and postoperatively for major surgical procedures, to assist in the healing of perineal or sacral wounds in patients with urinary incontinence and the relief of acute or chronic urinary retention. It may also be used in special circumstances such as in the improvement of comfort in palliative care. In some cases, catheters may be required for prolonged periods of time including spinal cord injury, though intermittent catheterisation is preferred [1]. In general, catheters are considered to be long-term when they have been in situ for 30 days or more [2].
Epidemiology of catheterisation
The prevalence of catheterisation varies internationally and between the acute, long-term institutional and community settings. A 2013 point prevalence survey by the European Centre for Disease Control and Prevention (ECDC) of 231,459 patients being cared for in 947 acute care hospitals in 29 countries showed that 17.2% were catheterised at the time of the survey, a figure that likely in its majority represents short-term catheterised patients (i.e. <30 days). This varied greatly between countries, ranging between 6.4% in Lithuania to 30.7% in Greece [3].
Away from the acute setting, a further ECDC survey of 77,264 patients in 1181 long-term institutions demonstrated a median prevalence of 6.3% catheterised, with the Czech Republic the highest of all 19 countries surveyed at 33.3% [4].
Patients in the community also represent a significant proportion of those catheterised. Surveying a large cohort (n=4010) receiving care at home in 11 European countries, Sørbye and colleagues [5] found that 11.5% of males and 3.3% of females had an indwelling urinary catheter. Again, differences in healthcare practice revealed variations between countries with higher prevalence in France, Germany and Italy.
Complications associated with catheterisation
Complications of catheterisation include catheter-associated urinary tract infection (CAUTI), direct urethral trauma, urethral stricture, non-infective urethral inflammation and impaired mobility [6]. Bladder tumours may also arise more frequently in patients with long-term chronic indwelling catheters due to catheter-induced chronic inflammation leading to malignant transformation [7]. CAUTI itself may also cause local complications including urethritis, prostate gland abscess and prostatitis [2].
Epidemiology and economic impact of CAUTI
Urinary tract infection (UTI) is an important cause of morbidity and mortality in the healthcare setting, accounting for 19% of all nosocomial infections. Of these, it is estimated that 43-56% are CAUTI [8]. If inadequately treated, CAUTI may progress to a bacteraemia and consequent urosepsis syndrome, multiplying risk of mortality and extending hospital stay [1]. Given such high prevalence, the economic implications of CAUTI are considerable; costing an estimated £1968 per patient episode and £99 million annually to the NHS, though sound economic analysis is still lacking [8].
Asymptomatic bacteriuria and the pathogenesis of CAUTI
Bacteriuria is defined as the “presence of bacteria in the urine revealed by quantitative culture or microscopy.” Between 2-7% of catheterised patients will acquire a bacteriuria every day despite best practice [9], with culture positive urine being effectively universal by 30 days [2].
Causative pathogens may contaminate the urinary tract via a variety of sources. Endogenous bacteria are typically meatal, vaginal or rectal commensals. Exogenous sources include the contaminated hands of patients and healthcare personnel as well as hospital equipment [1]. Though Escherichia coli is classically the most common pathogen, many other strains have been isolated including Pseudomonas aeruginosa, coagulase negative Staphylococcus and Candida species [2]. In long-term catheterised patients, two or more strains are commonly isolated [9].
When entering the urinary tract, pathogens may migrate extraluminally, via the outside of the catheter, or intraluminally through the catheter drainage system itself. There is little evidence to differentiate which route is more important in the pathogenesis of CAUTI, though the rapid decline in incidence following the introduction of closed catheter systems in the 1960s suggest the intraluminal route may be of greater significance [1].
Progression from bacteriuria to CAUTI occurs in around 24% of patients [8]. Risk factors for progression to CAUTI include female gender, advanced age, immunosuppression and failure to maintain a closed catheter system [9]. Of patients developing CAUTI, 4% will develop a severe complication such as bloodstream infection [8]. CAUTI constitutes 8% of all hospital acquired bacteraemia [9], with this figure rising to 50% in long-term healthcare facilities [2].
When does bacteriuria become CAUTI?
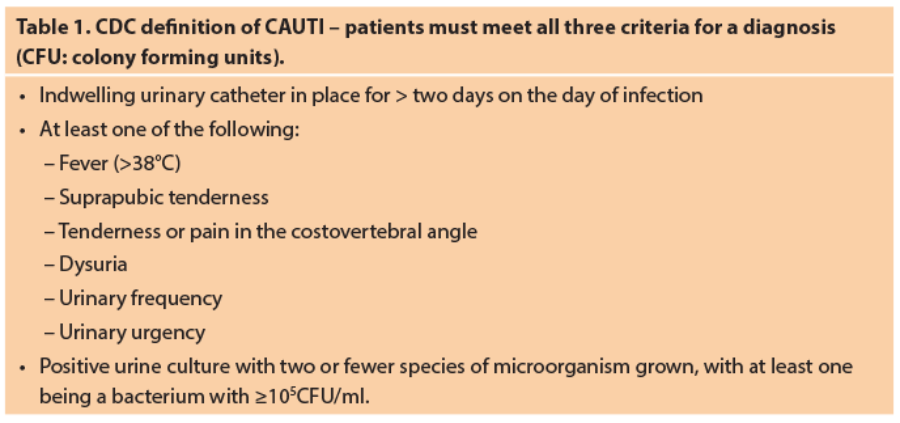
The definition of CAUTI remains controversial. The CDC has defined CAUTI as meeting all three of the criteria listed in Table 1 above [10].
The Infectious Disease Society of America (IDSA) definition differs slightly according to their published guidelines. The IDSA considers CAUTI as any UTI associated with a catheter in the presence of clinical features consistent with UTI, with no other identified sources of infection and a bacterial count of ≥103CFU/ml of ≥1 bacterial species in a single midstream or catheter specimen of urine [11].
Treatment of CAUTI
Treatment of CAUTI in the UK is governed by local hospital antimicrobial policy and antimicrobial sensitivities. Treatment should follow catheter replacement and should last seven days when there is a good response to treatment, or 10-14 days in cases in which there is a delayed response. Catheter change prior to commencing treatment is highly effective, with increased likelihood of cure or improvement at three days, decreased duration of fever and decreased likelihood of recurrence [9].
What are the challenges in the treatment of CAUTI?
Identification
Given the eventual ubiquity of bacteriuria in catheterised populations, differentiating CAUTI from other causes of hospital associated infection remains problematic. This is in part due to non-specificity of clinical presentation and common communication difficulties in affected patient populations (e.g. dementia or delirium) [6]. Diagnosis should be made taking into account the criteria set out in Table 1 and should not be guided by urine dipstick as this has little predictive value for differentiating asymptomatic bacteriuria from CAUTI. As repeated urine culture in catheterised patients may also lead to inappropriate diagnosis of CAUTI and consequent antibiotic treatment, it is recommended that catheter specimens of urine only be sent in the context of the above clinical signs [9]. In order to maintain a closed system, catheter specimens of urine should be collected via a dedicated port or direct needle puncture of the catheter tubing [2].
Antimicrobial defence mechanisms
Microbiological factors pose an enormous challenge. Microorganism biofilm formation due to extracellular polysaccharide secretion onto the catheter surface forms a protective barrier against antimicrobial agents and host defences [8], with mature biofilm formation usually seen after 14 days [2]. Without catheter removal, these are almost impossible to completely eradicate [1].
Emerging patterns of antimicrobial resistance are also a worrying trend. A large retrospective study by Cullen and colleagues [12] analysed 42,033 Escherichia coli isolates from hospital and community acquired UTI from 1999-2009 in Dublin, Ireland. 33.8% were resistant to trimethoprim; a common first-line empirical agent used both in Ireland and the UK. Against gentamicin, an agent often reserved for systemic infection, resistance grew by a rate of 0.7% per year over the study period with an overall resistance rate of 3.4%. In the United States, multi-drug resistant strains, defined as resistance to all agents in four or more classes, was seen in 4% of Pseudomonas aeruginosa and 9% of Klebsiella pneumoniae isolates [1]. Clearly antibiotic choice and stewardship vary greatly around the world, and consequently so do resistance patterns. Nevertheless, the combination of emerging resistance worldwide along with the defence afforded by biofilm formation are set to make successful treatment with systemic antibiotics ever more difficult to achieve.
Prevention of CAUTI: conservative measures
Prevention of CAUTI is therefore of utmost importance, with guidelines recommending that catheterisation be seen as a ‘method of last resort’. All alternatives should be explored prior to catheter insertion [8,9]. If unavoidable however, conservative measures including the rigorous application of aseptic technique on insertion, blockage prevention, perineal hygiene and maintenance of a closed system are simple but effective strategies with which all clinical hospital staff should be familiar [1]. Need for catheter should be reviewed on a daily basis, with removal instituted at the earliest possible opportunity [2]. It is unsurprising therefore that catheterisation is treated as a specialised procedure by the UK Royal College of Nursing (RCN), with extensive guidance being provided on preparation, insertion, catheter care and documentation [13].
What do we know from the current evidence about CAUTI prevention?
In addition to the conservative measures detailed above, many avenues of research have opened in an attempt to reduce the healthcare burden associated with CAUTI, with varying degrees of success. Evidence quality remains a challenge however, with heterogeneity of study design and results often preventing definitive conclusions being reached. In the following paragraphs, these issues are explored in more depth.
Antibiotic prophylaxis
Administration of prophylactic antibiotics for CAUTI prevention represents a logical potential intervention in CAUTI prevention. A review by Lusardi et al. [14] of six relevant trials found the use of antibiotic prophylaxis to be of benefit in reducing the rate of bacteriuria in surgical patients (RR 0.2, 95% CI 0.13 to 0.31) as well as signs of infection including pyuria and febrile morbidity. The authors urged cautious interpretation however; very little was reported on antimicrobial resistance, quality of life (QoL), adverse events and cost utility. This was echoed in a further review by Niel-Weise and colleagues [15] evaluating antimicrobial prophylaxis in long-term catheterisation (defined in this case as >14 days). Though there was some evidence that prophylaxis may be of use in intermittent catheterisation, inconsistent results meant it was difficult to draw meaningful conclusions overall. Both the CDC and the Scottish Intercollegiate Guidelines Network (SIGN) do not recommend the routine use of antibiotic prophylaxis in clinical practice [1,9].
Catheter route
Surprisingly little is known about the relative superiority of the different routes of catheterisation. Kidd and colleagues’ extensive review included 25 trials comparing suprapubic versus indwelling short-term catheterisation [16]. There was insufficient evidence to draw conclusions about incidence of CAUTI. Nineteen of the included trials assessed incidence of asymptomatic bacteriuria, finding increased risk for patients with indwelling catheters (RR 2.25, 95% CI 1.63-3.10), though this was deemed to be very low quality evidence. None of the studies reported ease of use, QoL or cost utility.
Change of catheter material
A further potential strategy would be to change the material from which the catheter is made. A recent, large multicentre trial by Pickard et al. sought to compare the standard Foley catheter versus silver-alloy and nitrofurazone-impregnated catheters [17]. Interestingly, as the latter only marginally reduced the incidence of CAUTI, this was not considered clinically important and patient discomfort was also a potential limiting factor in their use. The antiseptic silver-alloy catheters did not reduce incidence of CAUTI. Both catheters were also considerably more expensive. Clearly, the additional healthcare costs this would entail would render this non-viable in the current economic climate.
Intermittent catheterisation
As mentioned, intermittent catheterisation remains a widely used strategy for long-term bladder drainage solutions. Theoretically, micro-organisms are less likely to colonise the urinary tract as the catheter is only inserted at intervals and is removed after voiding is completed. This is typically indicated in neurogenic bladder dysfunction [16]. Potential complications include local skin irritation, ulceration, skin constriction and necrosis, occurring in around 15% of patients [18]. With regards to reduction of the incidence of CAUTI, evidence is inconclusive as to insertion technique, single versus multiple use catheters and material used [19]. Indeed, it is still unclear as to its benefits when compared with indwelling catheterisation in this context, despite a large number of relevant trials [17].
External catheterisation
When urinary incontinence is the main issue rather than urinary tract obstruction, external catheterisation may be of value. These are typically composed of either a latex or rubber sheath which is applied to the penis via an adhesive strip; so-called ‘condom catheters’. In addition to the greatly reduced risk of trauma, there is some evidence that condom catheters are associated with a decreased risk of asymptomatic bacteriuria and CAUTI compared with indwelling catheters, particularly in co-operative male patients [20]. An expert panel convened in 2016 to appraise the literature related to external catheterisation [21]. Multiple knowledge gaps were identified, in particular efficacy studies for CAUTI prevention and cost analyses. Clearly there is much scope for further research in this promising area.
Catheter-care bundles
Taking a systems approach, catheter-care ‘bundles’ have been advocated in which a range of measures are globally instituted within a healthcare institute to reduce CAUTI. These include healthcare staff education, guidelines for safe insertion and management as well as CAUTI surveillance. Such a bundle has been demonstrated to effectively decrease CAUTI rates by 37% in a study involving intensive care units in 15 developing countries [22]. The UK’s Department of Health (DoH) describes its catheter-care bundle as a ‘High Impact Intervention’ and provides an online form for continuing audit and training purposes [23].
Catheter washouts
In addition to progression to CAUTI, bacteriuria can lead to direct blockage of the catheter lumen. The build-up of encrustations (due to biofilm formation) can result in severe discomfort and urine bypassing the catheter via the extraluminal route. This is most commonly attributed to Proteus mirabilis. A rise in urinary pH due to its metabolism of urea to bicarbonate and ammonia leads to the formation of crystalline deposits including struvite (magnesium ammonium phosphate) and calcium phosphate [24]. Catheter washout has been posited as a solution to prevent this from occurring. An updated 2017 review by Shepherd and colleagues assessed trials comparing different washout policies in long-term catheterised adults including saline, acidic solution, antibiotic solution and no washout at all [24]. Due to methodological flaws and inadequate reporting of the seven included studies, it was not possible to conclude whether catheter washouts were beneficial or harmful and there were no data regarding patient satisfaction and discomfort.
Direction of future research
As discussed, the evidence base pertaining to catheterisation is limited with several critical outcomes including QoL and cost-utility remaining largely unstudied. Heterogeneous results have rendered meaningful conclusions in many areas difficult. The healthcare burden of catheter-related morbidity and cost highlight the urgency of this issue. As many review authors have concluded, further appropriately powered RCTs adhering to the CONSORT statement are required to address this.
The effect of catheterisation on patient QoL is likely to be extensive. Wilde et al. [25] derived a device-specific QoL instrument for adults with either indwelling or suprapubic long-term catheterisation based on the already validated generic Incontinence QoL (I-QOL) tool. This is unique in catheter research. As seen, there is a paucity of data regarding this outcome. Future studies must endeavour to address this.
Dahm and colleagues [26] have highlighted how the absence of high quality evidence is adversely affecting both frontline clinical care and the ability of professional organisations such as the European Association of Urology (EAU) and the American Urological Association (AUA) to disseminate guidelines. The authors have proposed a number of solutions for improving RCTs. These include the identification of areas of genuine clinical equipoise, assembly of multidisciplinary teams under the guidance of a principal investigator within an accredited clinical trials unit, use of feasibility to studies to determine the need for a full trial and involvement of key stakeholders (including urology patients) in all aspects of the trial design and execution.
Overall conclusions
Demand for healthcare has never been greater. This is in no small part due to an ageing population, along with increasingly complex diagnostic and treatment options. A well-developed understanding of what factors contribute to the prevention of CAUTI will both save lives and ease the enormous burden of catheter associated morbidity on healthcare services around the world. With pressure to find solutions mounting, opportunities for investigating CAUTI and related outcomes with high quality research are myriad in this expanding field.
References
1. Centers for Disease Control and Prevention Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections. 2009.
2. Nicolle L. Catheter associated urinary tract infections. Antimicrob Resist Infect Control 2014;3:23.
3. European Centre for Disease Prevention and Control. Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. 2013.
4. European Centre for Disease Prevention and Control. Point prevalence survey of healthcare associated infections and antimicrobial use in European long-term care facilities. 2013.
5. Sørbye LW, Finne-Soveri H, Ljunggren G, et al. Indwelling catheter use in home care: elderly, aged 65+, in 11 different countries in Europe. Age Ageing 2005;34(4):377-81.
6. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35(5):464-79.
7. Van Batavia J, Yamany T, Molotkov A, et al. Bladder cancers arise from distinct urothelial sub-populations. Nat Cell Biol 2014;16(10):982-91.
8. Loveday HP, Wilson JA, Pratt RJ, et al. UK Department of Health. epic3: national evidence-based guidelines for preventing healthcare-associated infections in NHS hospitals in England. J Hosp Infect 2014;86(Supplement 1):S1-S70.
9. Scottish Intercollegiate Guidelines Network. SIGN 88: Management of suspected bacterial urinary tract infection in adults. A national clinical guideline. 2012.
10. Centers for Disease Control and Prevention. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) and Other Urinary System Infection [USI]) Events. 2017.
11. Hooton TM, Bradley SF, Cardenas DD, et al. Infectious Diseases Society of America. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50(5):625-63.
12. Cullen IM, Manecksha RP, McCullagh E, et al. The changing pattern of antimicrobial resistance within 42 033 Escherichia coli isolates from nosocomial, community and urology patient-specific urinary tract infections, Dublin, 1999–2009. BJU International 2012;109(8):1198-206.
13. Royal College of Nursing (RCN). Catheter care: RCN guidance for nurses. 2012.
14. Lusardi G, Lipp A, Shaw C. Antibiotic prophylaxis for short-term catheter bladder drainage in adults. Cochrane Database Syst Rev 2013;(7).
15. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;(8).
16. Kidd EA, Stewart F, Kassis NC, et al. Urethral (indwelling or intermittent) or suprapubic routes for short-term catheterisation in hospitalised adults. Cochrane Database Syst Rev 2015;(12).
17. Pickard R, Lam T, MacLennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet 2012;380(9857):1927-35.
18. Golji H. Complications of external condom drainage. Paraplegia 1981;19(3):189-97.
19. Prieto J, Murphy CL, Moore KN, Fader M. Intermittent catheterisation for long-term bladder management. Cochrane Database Syst Rev 2014;(9).
20. Saint S, Kaufman SR, Rogers MA, et al. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc 2006;54(7):1055-61.
21. Gray M, Skinner C, Kaler W. External collection devices as an alternative to indwelling urinary catheter: Evidence-based review and expert clinical panel deliberations. J Wound Ostomy Continence Nurs 2016;43(3):301-07.
22. Rosenthal VD, Todi SK, Álvarez-Moreno C, et al. Impact of a multidimensional infection control strategy on catheter-associated urinary tract infection rates in the adult intensive care units of 15 developing countries: findings of the International Nosocomial Infection Control Consortium (INICC). Infection 2012;40(5):517-26.
23. Department of Health. High Impact Intervention: Urinary catheter care bundle. 2010.
http://webarchive.nationalarchives.
gov.uk/20120118164404/
hcai.dh.gov.uk/files/2011/03/
Document_-Urinary_Catheter_Care_High_Impact_
Intervention_FINAL_100907.pdf.
Last accessed 26 June 2017.
24. Shepherd AJ, Mackay WG, Hagen S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev 2017;(3).
25. Wilde MH, Getliffe K, Brasch J, et al. A new urinary catheter-related quality of life instrument for adults. Neurourol Urodyn 2010;29(7):1282-5.
26. Dahm P, N’Dow J, Holmberg L, Hamdy F. The Future of Randomised Controlled Trials in Urology. Eur Urol 2014;66(1):1-3.
Take Home messages
-
Catheter associated urinary tract infection represents a major burden to healthcare systems worldwide.
-
Challenges to treatment include identification of infection and antimicrobial resistance.
-
A number of methods have been investigated to prevent infection including catheter type, route of catheterisation and antimicrobial prophylaxis.
-
Overall the evidence base is of low methodological quality and very little is known about cost-effectiveness and quality of life.
-
Adequately powered, high quality, randomised controlled trials are required.
Declaration of competing interests: None declared.