Introduction
Clean intermittent self catheterisation (CISC) was first introduced and popularised by Lapides in 1972. Since then its utilisation has become widespread and it is now commonly used throughout the world as the preferred means of facilitating complete and effective bladder drainage in selected patient cohorts [1].
When performed correctly, CISC is a safe and effective technique but in common with many patient management techniques, it is often accompanied by a variety of potential, and perhaps unavoidable, complications. An understanding of these complications is vital not only for patient counselling prior to the introduction of the technique, but also for the effective prevention and management of its sequelae.
CISC is an excellent option to afford bladder emptying, both when the patient is unable to achieve any intentional voiding or when the patient voids intentionally, but incompletely, leaving a significant residual volume; it can be performed by both patient and carer alike. This article aims to review the available evidence pertaining to the potential complications of CISC.
Complications
Infection
Catheter associated urinary tract infection (CAUTI) is estimated to be the most common healthcare associated infection (HAI) in the world and the second most prevalent in England. In 2011 there was a HAI rate of 6.4% and UTIs accounted for 1.72%, almost all attributable to instrumentation of the urinary tract [2]. However, the specific rate of CAUTI secondary to CISC remains unclear. Nevertheless, it is the most common and serious complication for a patient performing CISC with an almost universal lifetime incidence in those performing the technique.
Inconsistent definitions of bacteriuria and urinary tract infection in the literature make it difficult to discern the exact incidence, prevalence and relative risk amongst the varying patient cohorts utilising this technique as a means of bladder drainage. Studies have suggested rates of approximately 2.6% per year cumulating in a five-year incidence in excess of 80% [3,4,5]. Evidence does suggest there is a benefit (in terms of UTI incidence) favouring gel reservoir and hydrophilic coated catheters – but cost is a prohibitive factor. The European Association of Urology guidelines recommend an aseptic technique as a compromise.
A recent Cochrane review on urinary catheter policies for long-term bladder drainage found that there was minimal difference in the rates of CAUTIs in patients performing CISC, regardless of whether patients were taking prophylactic antibiotics or not. This minimal but statistically significant difference was only apparent when comparing rates of afebrile UTI (incidence density relative rate 0.69), whilst no statistically significant difference was found when comparing rates of febrile UTIs [6]. Two older studies, both in paediatric populations, provide conflicting evidence, with the larger finding a higher rate of symptomatic UTIs in CISC patients who were given prophylactic antibiotics [7,8]. Another Cohrane review [9] found no benefit in the use of cranberries in juice or supplement form in preventing urinary tract infections in patients performing CISC, in contrast to those not performing CISC in whom cranberry supplementation reduces the incidence of UTI.
Table 1: Risk factors for UTI.
Decreased frequency of catheterisation
Decreased frequency of catheterisation increases the time urine is stagnant in the bladder, allowing bacterial proliferation. It also leads to overfilling – stretching of the bladder wall and occlusion of capillary flow thus reducing the availability of vital immune substrates.
Increased frequency of catheterisation
Increased frequency, especially in the setting of poor technique, increases the rate at which bacteria are introduced into the bladder and thus increases the risk of UTI.
Excessive fluid intake
Increased fluid intake leads to overdistention of the bladder and decreased delivery of immune substrates, or may result in increased CISC frequency.
Decreased fluid intake
Decreased fluid intake leads to more concentrated urine and encourages a decrease in the rate of performing CISC.
Poor technique
Poor technique leads to the introduction of bacteria and increased likelihood of urethral trauma.
Pain
Pain secondary to poor technique or urethral trauma is a disincentive to continuing CISC and may result in infrequent catheterisation or complete non-compliance.
Nocturnal polyuria
Overfilling of the bladder during sleeping hours causes bladder distension, increased stagnation, inefficient voiding and decreased delivery of immune substrates.
There are many risk factors associated with the incidence of UTI amongst patients who intermittently catheterise (Table 1), of which the most influential is thought to be technique. An excellent technique significantly reduces the risk of UTI and is one of the main indications for yearly patient consultation and technique review.
Treatment is with an appropriate antibiotic (and based according to culture, sensitivity, previous efficacious therapy and local policy).
Pyelonephritis
CISC related pyelonephritis is rare and poorly documented in the literature, with available studies suggesting a rate of 5% over six months in patients newly taught to perform CISC one to four times per 24 hours [4]. Management is with appropriate fluid and antibiotic therapy according to culture, sensitivity, previous efficacious treatment and local policy.
Urethritis
Urethritis is infrequent in the modern era as a direct consequence of improved lubrication and significantly less urethral trauma, but historical rates of between 2-19% have been reported [10-12]. It is likely that these rates have declined in recent years, with the advent of newer catheter types, materials and training programmes, etc. There is some evidence to suggest hydrophilic catheters are superior to non-coated catheters, demonstrating reduced cytological evidence of inflammation – although a direct correlation between this and urethritis has yet to be conclusively shown [13].
Epididymo-orchitis
Epididymo-orchitis is reported to occur in the region of 2.5-10% in the short term, increasing in the long term to over 40% [4,14,15]. Treatment is again with appropriate antibiotic therapy according to culture sensitivity and local policy, usually for a prolonged period of between five to six weeks.
Prostatitis
This complication is relatively common in patients performing CISC, with rates reported between 8 and 31% [16,17]. Patients typically present with perineal pain and in particular pain on catheterisation with or without a febrile illness. Treatment is with appropriate antibiotic therapy usually for a period of between five to six weeks, along with good hydration, adequate analgesia and on-going supportive therapy as required. Suprapubic catheterisation is often required and in very rare cases, progression to a prostatic abscess may occur requiring drainage, either transrectally or transurethrally.
Trauma
Irritation, inflammation and trauma to the urethra due to CISC are common occurrences with a long-term incidence of recurrent urethral bleeding in the region of 29%. Although much lower rates of urethritis (2-19%) have previously been quoted, many clinicians believe that some degree of urethral trauma is inevitable at some stage during a patient’s CISC career [10-12,18]. Potential causes for this include poor technique and / or anatomical knowledge, lack of lubrication during insertion and catheters adhering to the urethra on removal (again secondary to inadequate lubrication). A significant number of patients will experience bleeding during the early weeks following CISC initiation.
False passage
Almost certainly under reported, false passages occur as a result of trauma, most commonly at the distal aspect of the prostate. Patients experiencing false passages often find themselves or their carers incapable of adequately catheterising due to the catheter preferentially entering the false passage. The temporary use of a wider gauge catheter may circumvent the issue as a larger catheter is less likely to enter a smaller false passage. If unsuccessful, alternative means of effective bladder drainage, e.g. long-term urethral or supra-pubic catheterisation must be employed until the false passage has resolved and CISC can be recommenced.
Urethral stricture
The incidence of urethral stricture disease secondary to CISC increases with duration with prevalence rates ranging from 5% at 2.5 years to approximately 20% at 7 to 12 years [14,16,19]. Urethral stricture formation is associated with both the increased catheterisation rate and the number of years performing CISC; from this it could be inferred that risk of urethral stricture formation increases with overall number of times catheterised [16].
Meatal stenosis
Meatal stenosis secondary to CISC is a rare complication – although is a common indication for intermittent urethral dilatation. One older review article suggests a rate as high as 4% over a mean of seven years in a patient group of 75 utilising CISC for effective bladder drainage [16].
Treatment is either via continued CISC, or in more severe cases formal urethral dilatation or meatoplasty.
Bladder perforation
Bladder perforation is an exceptionally rare complication of CISC, although has been reported both in the neurogenic and the augmented bladders [20-25]. Bladder necrosis has similarly only been highlighted in case reports [20]. Treatment is with antibiotic therapy and either laparotomy and repair with a period of indwelling catheterisation for intraperitoneal rupture or drainage and indwelling catheterisation for extraperitoneal perforation.
Miscellaneous
Catheter knotting
Ball et al., Guérin et al., Brown et al. and Dogra et al. have all produced case reports on the rare complication of catheter loss into the bladder and subsequent knotting [26-29]. It is difficult to determine risk factors or frequency for this rare event.
Calculi
The incidence of bladder calculi formation in children and adults who perform CISC is higher than that of the general population. Bladder calculi are particularly well documented in adults with spinal cord injury performing CISC [30]. Ord et al. retrospectively found a bladder calculi rate of 1.5% (one patient) in their 71 patient CISC managed subset of spinal cord injury patients over a median follow-up of 81 months (giving an absolute annual risk of approximately 0.2%) [31]. More recently, rates as high as 15% over 17 years have been reported in spinal cord injury patients performing CISC [15]. The most common genesis of such bladder calculi is the introduction of pubic hair into the bladder as a consequence of retrograde catheter passage into the bladder, which then acts as a nidus for stone formation [32-34].
Renal calculi appear to be more common in patients performing CISC although the literature focuses exclusively on those patients with spinal cord injuries, thus the quoted rates of 2-6% at five years and 9% at 17 years may not be directly transferable to the entire population of patients performing CISC [15,35]. The recurrence rate in this patient group is high, with approximately 25% forming a second stone within five years [36].
Pain
Although CISC itself generally improves quality of life (QoL), in more than 60% of patients, severe chronic pain whilst performing CISC is a major predictor for a patient having a poor QoL [36]. Pain as a complication of CISC is universal initially, and may be secondary to a number of causes including: poor technique / trauma, UTI, stone disease and / or bladder spasm. When assessing pain during insertion or removal of the catheter, consideration needs to be given to failure of the pelvic floor muscles to relax, mucosal atrophy (in older women) or the patient not occluding the catheter as it is withdrawn – the subsequent vacuum sucking the bladder wall and urethra into the catheter eyelets.
Fear and anxiety are important contributing factors to the pain experienced when performing CISC, both of which increase muscle contraction and hinder a patient’s education (delaying improvement of technique) propagating pain. Adequate training of the patient / carer by an appropriately trained and empathic healthcare professional greatly ameliorates these factors.
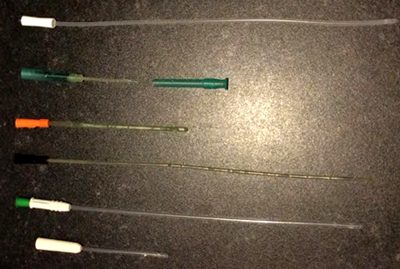
Figure 1: From top to bottom: HydroSil Gripper; SpeediCath Compact;
SpeediCath Female; SpeediCath Male; LoFric Sense; LoFric Origo.
Previously, there was not enough evidence to favour any particular type of catheter for CISC, although there is now a growing body of research that suggests newer hydrophilic coated catheters may reduce both rates of urinary tract infection and urethral inflammation caused by CISC and thus, it is surmised, the incidence of trauma-related sequelae. Currently, there is limited evidence favouring hydrophilic catheters with respect to, in varying degrees, urinary tract infection rates, cytological appearance of disruption to the urethra, haematuria and patient satisfaction [13,38-40].
It is important however, to note that the current evidence base has not been deemed enough by the National Institute for Health and Care Excellence (NICE) to recommend the use of the significantly more expensive hydrophilic-coated and gel reservoir catheters over their non-coated, cheaper, alternatives. NICE guidelines were unable to make any formal recommendation with regard to the best choice of catheter, noting that of the four groups assessed (single use hydrophilic-coated, single use gel reservoir, single use non-coated and multiuse non-coated), from a cost-effectiveness analysis and quality of life standpoint, when balanced against complications (albeit with a highlighted dearth of evidence), the best option would be reusable non-coated catheters, but non-coated catheters are not licensed for multiple use, only as single use disposable item [41]. The lack of evidence precludes NICE from making an ‘off-licence’ recommendation.
Similarly, a recent cost-benefit analysis of CISC found that although gel reservoir and hydrophilic-coated catheters did significantly decrease the incidence of UTIs (by approximately 150 per 1000 for both), there was no difference detected in monthly or yearly UTI rates. (It is also worth noting there was no significant difference in rates detected between a clean or sterile technique for passing a non-coated catheter.) Although this suggests that hydrophilic or gel reservoir catheters are the best option, the cost is significantly higher (£2339 or £2482 per annum) than CISC (£864 per annum, using one catheter per day), but almost half that of sterile use of non-coated catheters (£4343 per annum) [42].
Conclusion
Whilst intermittent self (or carer administered) catheterisation is an excellent means of achieving effective bladder drainage, it is not without its complications, all of which must be discussed when counselling a patient prior to the introduction of the technique to the patient’s management regimen. Newer catheter types, coating and lubrication may reduce the incidence of complications, but are not currently cost-effective in the current era.
References
1. Lapides J, Diokno AC, SilberSJ, Lowe BS. Clean, intermittent self-catheterization in the treatment of urinary tract disease. Journal of Urology 1972;107(3):458–61.
2. Health Protection Agency. English National Point Prevalence Survey on Healthcare Associated Infections and Antimicrobial Use, 2011: Preliminary data. London, UK; Health Protection Agency; 2012.
3. Biering-Sorensen F, Nielans HM, Dorflinger T, Sorensen B. Urological situation five years after spinal cord injury. Scand J Urol Nephrol 1999;3:157-61.
4. Turi MH, Hanif S, Fasih Q, Shaikh MA. Proportion of complications in patients practicing clean intermittent self-catheterization (CISC) vs indwelling catheter. JPMA 2006;56(9):401-4.
5. Woodbury MG, Hayes KC, Askes HK. Intermittent catheterization practices following spinal cord injury: a national survey. Can J Urol 2008;15(3):4065-71.
6. Niel-Weise BS, van den Broek PJ, da Silva EMK, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev 2012;(8):CD004201.
7. Johnson HW, Anderson JD, Chambers GK, et al. A short-term study of nitrofurantoin prophylaxis in children managed with clean intermittent catheterization. Pediatrics 1994;93(5):752–5.
8. Schlager TA, Anderson S, Trudell J, Hendley JO. Nitrofurantoin prophylaxis for bacteriuria and urinary tract infection in children with neurogenic bladder on intermittent catheterization. Journal of Pediatrics 1998;132:704–8.
9. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev 2012;(10):CD001321.
10. Lapides J, Diokno AC, Gould FR, Lowe BS. Further observations on self-catheterization. J Urol 1976;116:169-72.
11. Wyndaele JJ, Oosterlinck W, De Sy W. Clean intermittent self-catheterization in the chronic management of the neurogenic bladder. Eur Urol 1980;6:107-10.
12. Labat JJ, Perrouin-Verbe B, Lanoiselee JM, et al. L’autosondage intermittent propre dans la réeducation des blesses medullaires et de la queue de cheval I + II. Annales de Réadaptation et de Médicine Physique 1985;28:125-36.
13. Vaidyanathan S, Soni BM, Dundas S, Krishnan KR. Urethral cytology in spinal cord injury patients performing intermittent catheterisation. Paraplegia 1994;32:493–500.
14. Perrouin-Verbe B, Labat JJ, Richard I, et al. Clean intermittent catheterisation from the acute period in spinal cord injury patients. Long term evaluation of urethral and genital tolerance. Paraplegia 1995;33(11):619-24.
15. Ku JH, Jung TY, Lee JK, et al. Risk factors for urinary stone formation in men with spinal cord injury: a 17-year follow-up study. BJU Int 2006;97(4):790-3.
16. Wyndaele JJ, Maes D. Clean intermittent self-catheterization: a 12 year follow-up. J Urol 1990;143:906-8.
17. Wyndaele JJ. Chronic prostatitis in spinal cord injury patients. Paraplegia 1985;23:164-9.
18. Webb R, Lawson A, Neal D. Clean intermittent self-catheterization in 172 adults. Br J Urol 1990;65:20-3.
19. Kuhn W, Rist M, Zaech GA. Intermittent urethral self-catheterisation: long term results (bacteriological evolution, continence, acceptance, complications). Paraplegia 1991;29(4):222-32.
20. Reisman EM, Preminger GM. Bladder perforation secondary to clean intermittent catheterization. J Urol 1989;142(5):1316-17.
21. Ubrig B, Waldner M, Gleibner J, Roth S. Retrovesical hematoma secondary to clean intermittent self-catheterization. J Urol 2001;166(2):616.
22. Fabricius MJ, McInerney PD, Lambert AW. Perforation of the native urinary bladder as a complication of intermittent self catheterization. BJ Med Surg Urol 2011;4(2):89-90.
23. Elder JS, Snyder HM, Hulbert WC, Duckett JW. Division of Urology, Children’s Hospital of Philadelphia, Pennsylvania. J Urol 1988;140(5,2):1159-62.
24. Leibovitch I, Ramon J, Ben Chaim J, Goldwasser B. [Delayed spontaneous rupture of the bladder following augmentation enterocystoplasty.] Harefuah 1990;119(3-4):68-70.
25. Hasan ST, Marshall C, Robson WA, Neal DE. Clinical outcome and quality of life following enterocystoplasty for idiopathic detrusor instability and neurogenic bladder dysfunction. BJ Urol 1995;76:551–7.
26. Ball RA, Horton CE, Mandell JA. Transurethral removal of knotted bladder drainage catheter in a male following bladder neck reconstruction. Urology 1993;41(3):234-6.
27. Guérin JG, Marie L, Sibert L, et al. [Unusual complication of self-catheterization in a patient with a continent stoma]. Prog Urol 1996;6(3):434-5.
28. Brown SC, Lynn NN. An unusual (knotty) complication of clean intermittent self-catheterization in a patient with an augmented bladder. BJU Int 1999;84(4):539.
29. Dogra PN, Nabi G, Goel R. Endoscopic Removal of Knotted Urethral Catheter: A Point of Technique. Urol Int 2003;71:8-9.
30. Chen Y, DeVivo MJ, Lloyd LK. Bladder stone incidence in persons with spinal cord injury: determinants and trends, 1973-1996. Urology 2001;58(5):665-70.
31. Ord J, Lunn D, Reynard J. Bladder Management and Risk of Bladder Stone Formation in Spinal Cord Injured Patients. J Urol 2003;170(5):1734-7.
32. Kutscher HA, Vinson RK. Bladder calculi formation during clean intermittent catheterization. Urol 1979;14(4):357-8.
33. Solomon MH, Foff SA, Diokno AC. Bladder calculi complicating intermittent catheterization. J Urol 1980;124:140-1.
34. Amendola MA, Sonda LP, Diokno AC, Vidyasagar M. Bladder calculi complicating intermittent clean catheterization. A J Roentgenol 1983;141:751-3.
35. Chen Y, DeVivo MJ, Roseman JM. Current trend and risk factors for kidney stones in persons with spinal cord injury: a longitudinal study. Spinal Cord 2000;38:346–53.
36. Chen Y, DeVivo MJ, Stover SL, Lloyd LK. Recurrent kidney stone: a 25-year follow-up study in persons with spinal cord injury. Urology 2002;60:228–32.
37. Kessler TM, Ryu G, Burkhard FC. Clean intermittent self-catheterization: a burden for the patient? Neurourol Urodyn 2009;28(1):18-21.
38. Stensballe J, Looms D, Nielsen PN, Tvede M. Hydrophilic-coated catheters for intermittent catheterisation reduce urethral micro trauma: a prospective, randomised, participant-blinded, crossover study of three different types of catheters. Eur Urol 2005;48(6):978-83.
39. De Ridder DJ, Everaert K, Fernández LG, et al. Intermittent catheterisation with hydrophilic-coated catheters (SpeediCath) reduces the risk of clinical urinary tract infection in spinal cord injured patients: a prospective randomised parallel comparative trial. Eur Urol 2005;48:991–5.
40. Cardenas DD, Moore KN, Dannels-McClure A, et al. Intermittent catheterization with a hydrophilic-coated catheter delays urinary tract infections in acute spinal cord injury: a prospective, randomized, multicenter trial. PM & R 2011;3(5):408-17.
41. NICE clinical guideline 139. Infection: Prevention and control of healthcare-associated infections in primary and community care. March 2012.
42. Bermingham SL, Hodgkinson S, Wright S, et al. Intermittent self catheterisation with hydrophilic, gel reservoir, and non-coated catheters: a systematic review and cost effectiveness analysis. BMJ 2013;346:e8639.
Declaration of Competing Interests: None declared.









