Introduction
There has undoubtedly been a dramatic increase in the number of patients diagnosed with small renal masses in recent years [1]. The rapidly expanding use of CT has led to a large number of incidental diagnoses, but increasing longevity and the decreasing mortality from cerebrovascular and cardiovascular disease has almost certainly played a further part in the rise in incidence. As expected the majority of these lesions are discovered in patients over 60 years of age (Figure 1).
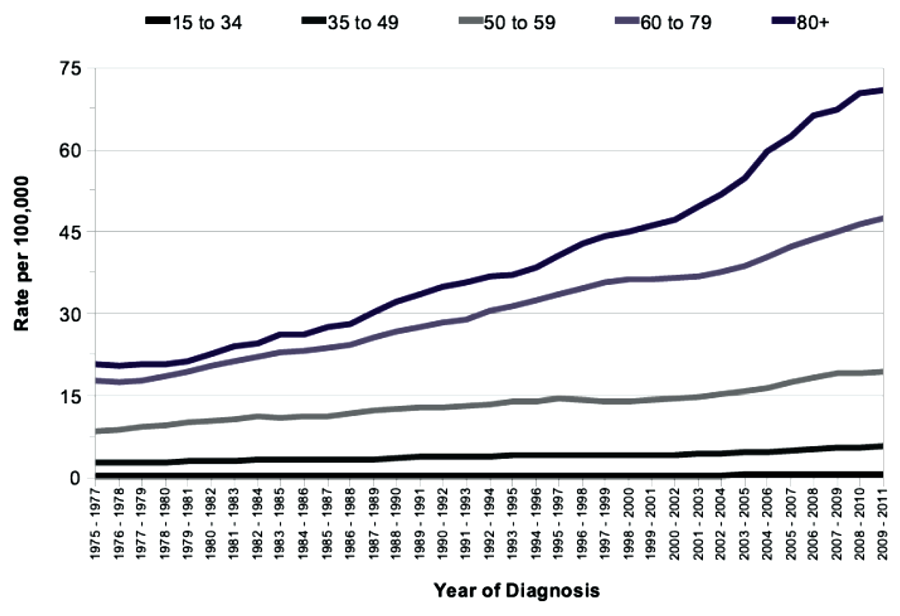
Figure 1: Age-standardised incidence rates per 100,000 population, by age.
This has left the urological community with the challenge of formulating management plans for these patients, and coping with the increased volume of work. The strategy at our centre (University Hospital Southampton) has included an increase in active surveillance along with utilisation of our expertise in image guided thermal ablation within our radiology department. With the help and support of our urological surgeons we have treated and carefully monitored over 300 tumours with percutaneous cryoablation. This represents one of the largest treatment cohorts in Europe.
Case selection
Of sub-4cm renal tumours some 20-30% will prove benign [2,3]. Whilst there is some debate regarding the exact place of surveillance, there is undoubted renewed interest in the use of renal biopsy to help stratify risk [4,5]. Renal biopsy appears to be increasingly reliable (audit has revealed a 7% indeterminacy rate against a mean tumour size of 31mm in our centre) and when coupled with tumour size and patient demographics allows both the clinician and patient to make a more informed decision regarding malignant potential and metastatic risk. This permits a variety of management options, which can be discussed with the patient. If definitive treatment is the chosen path there are still choices to be made, and with smaller volume disease thermal ablation is often one of those options chosen by the patient.
What is thermal ablation?
Thermal ablation describes several techniques that utilise extremes of temperature to destroy tissue. Radiofrequency ablation (RFA) and microwave ablation (MWA) are the most commonly used ‘hot’ techniques. In contradistinction cryoablation uses ice to cause tissue destruction. RFA has been the forerunner in establishing image-guided ablation as a viable technique and remains the most commonly used energy. The published data suggest it is effective if confined to <4cm tumours and probably most suited to tumours less than 3cm in diameter [6]. Tissue ablative technologies, and their guidance and control, have been refined over recent years.
Cryoablation has one of the longest track-records and the more recent evolution of third generation, argon-based systems has matured the technology to a highly effective surgical tool. In this respect cryoablation is making a strong case as the optimal ablative technique for treating renal tumours and seems better suited than RFA for more substantive tumours.
How does percutaneous cryoablation work?
Under imaging guidance a series of cryoprobes are placed into the target lesion with the patient in the prone oblique position. Often this is performed under general anaesthetic with CT guidance, and this is our method of choice. We believe CT offers the most accurate depiction of the tumour and treatment boundaries in all planes, and permits precise probe placement with tolerances of 3-4mm. MRI is also set to evolve as an effective and robust guidance tool in the next few years. The size and morphology of the tumour dictates the number of probes required and their configuration (Figure 2). Broadly speaking, the probes should be placed approximately 1cm from the tumour edge and less than 2cm apart.
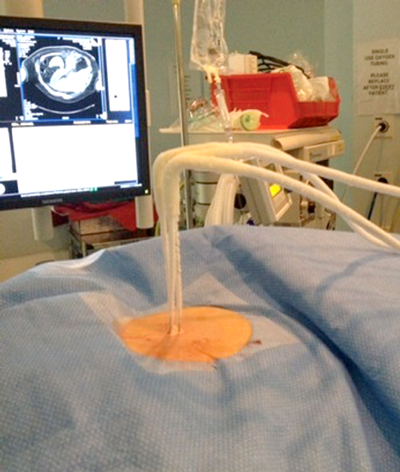
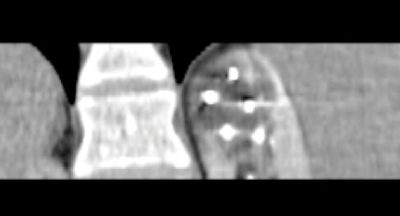
Figure 2: Probe placement.
Once the probes are appropriately positioned it may be necessary to protect vulnerable adjacent structures, such as the bowel, pancreas or ureter. The adjunctive techniques most commonly employed to achieve this protection involve the injection of fluid (hydrodissection) or gas (air dissection) to displace at risk structures (Figure 3).
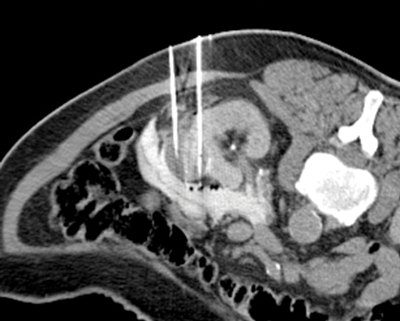
Figure 3: Peri-treatment image depicting cryoprobes within the iceball
and a protective layer of contrast tinted hydrodissection.
In addition the tumour can be manually levered away from the ureter once the tumour has frozen to the probes (commonly termed a ‘stickshift’ or ‘stickfreeze’). Once the probes are positioned our standard double freeze-thaw treatment cycle consists of a 10-minute freeze, 8-minute thaw, and a second 10-minute freeze. The evolving ice ball is visible on CT and imaged during the treatment cycle where it should be seen to consume the target tumour with three-dimensional treatment margins of at least 5mm. Critically, the radiologist has direct control of the power of each probe allowing manipulation of the size and morphology of the ablation zone as it forms.
The ice ball produces temperatures below -100 degree Celsius, and causes tissue destruction by both direct and indirect effects. Ice crystals initially form in the extracellular space causing dehydration, cell shrinkage and membrane damage. Further cooling leads to intracellular ice crystals which at temperatures below -30 to -40 degrees Celsius are almost always lethal [7]. The whole process lasts approximately 90-120 minutes, and our preference is to perform all procedures under a general anaesthetic. This is not because it is painful but because it permits optimal patient positioning and control over respiratory motion.
How do we know if it has been effective?
We know from experimental data that a circumferential treatment margin of 5mm is the bare minimum and routinely aim for visible ice at least 10mm beyond the tumour’s edge. The ice ball edge and hence the zero isotherm can be actively monitored during the procedure so as to confirm treatment adequacy. However, the final assessment of primary treatment success is made on follow-up imaging. We choose to perform a contrast enhanced CT at two weeks post procedure, which has been validated as a robust and consistent surrogate of primary treatment success.
Other centres use different approaches but essentially we are looking for an ablation zone that subsumes the original location of the tumour and a lack of residual contrast enhancement. Currently our confidence in that initial two-week assessment usually results in a follow-up plan of CT at one year, three years and five years post procedure. Clearly, any equivocal findings at two weeks would prompt an earlier interval scan at three months, or further definitive treatment, but negligible subtotal treatments are encountered with diligent technique.
Residual tumour or locally recurrent tumour is most commonly depicted as an area of nodular enhancement at the tumour margin (Figure 4).
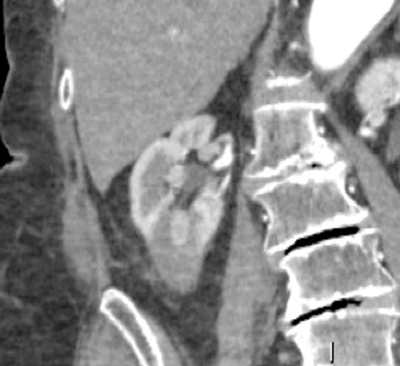
Figure 4: Nodular tumour recurrence within a lower pole ablation zone.
Primary treatment failure is now rare and reflects an undoubted ‘learning curve’ as with any other operative procedure. Despite an increasing case complexity we have had only two confirmed subtotal treatments in the last 100 treated tumours at Southampton. We believe any ablation service should now be aiming at a primary subtotal treatment rate of less than 5%, as an absolute maximum. When residual disease or even recurrent disease is rarely identified it is almost always amenable to retreatment with cryoablation.
Where does ablation fit in?
The most recent National Institute for Health & Care Excellence (NICE) guidance from 2011 states that the “evidence on the efficacy and safety of percutaneous cryotherapy for renal cancer is adequate to support the use of this procedure”. It suggests the other treatment options are partial or total nephrectomy (laparoscopic or open), or RFA. NICE also recommend an upper size limit of 4cm, but acknowledged the recent use of cryoablation in larger tumours.
The British Association of Urological Surgeons (BAUS) include cryoablation in its guidance document on renal cancer. They note the potential for reduced morbidity, a shorter hospital stay and the ability to treat patients unsuitable for surgery, including the elderly. In this statement BAUS have acknowledged the major advantages of percutaneous ablative techniques.
It should be recognised that much of the published literature on cryoablation is based on experiences in often poor surgical candidates. Inevitably this includes the elderly and significantly comorbid. Subsequently any coarse meta-analysis of outcome data between patients suitable for nephron sparing surgery and those possibly only suitable for cryoablation is fraught with bias, and there have been no completed randomised controlled trials to date. As experience with cryoablation develops and registries mature we may be able to make more evidence-based recommendations between the two groups. Our own prospective dataset is much less influenced by selection bias and we would argue that our outcomes are in equipoise to that of surgery. Whilst that claim may not be borne out in all centres, it seems beyond doubt that cryoablation has an impressively low complication rate and that the length of stay is consistently lower than that of surgical resection; almost all of our patients only stay one precautionary night post-procedure.
Apart from the high-risk surgical candidates, cryoablation is also ideally suited for patients with bilateral tumours, those with congenital disorders leading to a propensity for renal cell carcinoma, and in treating locally recurrent disease, following surgery or ablation, often including metastatic disease.
In Southampton almost all patients with a small renal mass suitable for cryoablation have this treatment option put to them. It should be noted that following multidisciplinary team (MDT) review the discussion of treatment options is undertaken by a urologist who provides an unbiased opinion regarding the relative merits and drawbacks of each technique; commonly cryoablation versus nephron sparing surgery for smaller volume disease.
So, what does all this mean?
It seems sensible that minimally invasive, low morbidity techniques should play a part in treating an increasing number of small renal masses in an ageing population. Whilst RFA is the most commonly employed ablative technology, cryoablation is establishing itself as the most elegant and effective image-guided treatment modality currently available. With oncological outcomes similar to those of surgery, a short length of stay, and a favourable complication profile, both patients and hospital managers are likely to support it.
That does not mean that we envisage cryoablation being available in every UK hospital, nor would we advocate it. Clearly there is a significant learning curve and the more complex procedures require considerable experience amongst the team including the radiographers and anaesthetists. In the event of postoperative problems, the supervising urologist needs a clear understanding of the technique and potential pitfalls. Similarly the radiologist will need to be aware of the often complex post-procedural imaging characteristics. For example, the deliberate addition of contrast-tinted fluid into various intraperitoneal and retroperitoneal spaces can be confusing, and the presence of apparently free gas used for dissection purposes can be alarming. For these reasons, and to ensure the best oncological and procedural outcomes, we feel cryoablation should only be available in centres able to maintain sufficient treatment volumes.
TAKE HOME MESSAGE
-
Suitable for almost all small renal masses in almost all patients.
-
Short hospital stay (usually one night post procedure).
-
Favourable complication rate.
-
Direct monitoring during treatment allows adaptation of the treatment zone and accurate assessment of margins.
-
Can be used to easily treat residual or recurrent disease.
The urological perspective
I would agree that in Southampton percutaneous cryoablation is equivocal to nephron sparing surgery with the proviso that longer outcome data is not yet available. What patients require is expert appropriate treatment. This requires a degree of centralisation so that the volume of patients treated by an interventional radiologist or a urological surgeon is sufficient to maintain the necessary skills. It is counterintuitive that newer RFA services are starting to ablate small lesions when the largest volume centre in Europe has abandoned RFA for renal disease in favour of cryotherapy. It is equally counterintuitive to believe the outcomes of the occasional laparoscopic partial nephrectomist are equivalent to a higher volume unit.
The second challenge is when not to treat. Very few doubt the indolent nature of many small renal lesions and many acknowledge the difficulty of predicting life expectancy. The hardest age group is the reasonably fit over 75-year-olds. Urologists are well placed, given our very similar experience with prostate cancer, but better prognostic tools are still sought after.
The key will be accurate data to inform patients, surgeons and radiologists. This will include information on size, location, pre and post renal function along with short and longer term outcomes. That will allow genuine patient choice and allow the treating urologist to guide that choice with evidence.
My opinion is that there will be a bias towards cryoablation, in units able to offer it, for smaller lesions and for the less fit. As partial nephrectomy centralises the bias will switch towards surgery as the size increases above 3-4cm, and will be offered to increasingly larger lesions. For patients with a good contralateral kidney the actuarial gains for partial nephrectomy are only apparent at 10 to 15 years and therefore we should not forget that nephrectomy may be a very valid alternative.
References
1. http://www.cancerresearchuk.org/
cancer-info/cancerstats/
types/kidney/incidence/uk-kidney-cancer-
incidence-statistics#trends
Last accessed October 2014.
2. Rosenkrantz AB, Wehrli NE, Melamed J, et al. Renal masses measuring under 2cm: pathologic outcomes and associations with MRI features. European Journal of Radiology 2014 [Epub ahead of print].
3. Kang SK, Huang WC, Pandharipande PV, Chandarana H. Solid renal masses: what the numbers tell us. American Journal of Roentgenology 2014;202(6):1196-206.
4. Phé V, Yates DR, Renard-Penna R, et al. Is there a contemporary role for percutaneous needle biopsy in the era of small renal masses? BJU Int 2012;109(6):867-72.
5. Menogue SR, O’Brien BA, Brown AL, Cohen RJ. Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int 2013;111(4 Pt B):E146-51.
6. Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. European Urology 2013; 63(3):486-92.
7. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998;37(3):171-86.
Declaration of competing interests: None declared.







