Non-invasive and invasive electro-stimulation techniques have been extensively studied in the treatment of lower urinary tract and bowel dysfunction, including overactive bladder syndrome (OAB), non-obstructive chronic urinary retention, faecal incontinence and chronic pelvic pain.
Currently, the most common indication for neuromodulation is OAB, which is defined by the International Continence Society (ICS) as urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology. OAB is a common, disabling condition, which affects about 60 million people in the USA [1] and neuromodulation techniques are recommended as third-line therapy – after behavioural and medical treatment has failed. At this time there are a number of new promising technologies actively being developed for this population.
This review is focused on neuromodulation techniques for patients with lower urinary tract dysfunction, including percutaneous tibial nerve stimulation, sacral neuromodulation and the Brindley procedure. Standard techniques, recent advances and new devices on the horizon are comprehensively described.
Neuromodulation techniques for idiopathic OAB
Tibial nerve stimulation
Percutaneous tibial nerve stimulation (PTNS) is a minimally invasive office-based procedure, which has been shown to be safe and effective in treating OAB symptoms [2]. As a prerequisite, the patients must be able to make frequent hospital / clinic visits to receive the therapy, making it time consuming for the patient and costly for both the patient and healthcare system. Most percutaneous TNS protocols suggest 12-weekly treatments followed by less frequent treatments to sustain OAB symptomatic improvement. Taking the current drawbacks into consideration, newer technologies for PTNS have been developed to provide implanted TNS systems, which could be used by the patient in their home environment.
A number of implantable TNS devices are being studied for OAB treatment. The Bioness Stimrouter™ neuromodulation system is one such device and can be implanted under local anaesthesia. The device consists of an implanted lead with an integrated receiver, anchor, and three electrode contacts in close association to the posterior tibial nerve. The lead captures stimulation energy delivered wirelessly transdermally from an external pulse transmitter and electrode patch (Figure 1).
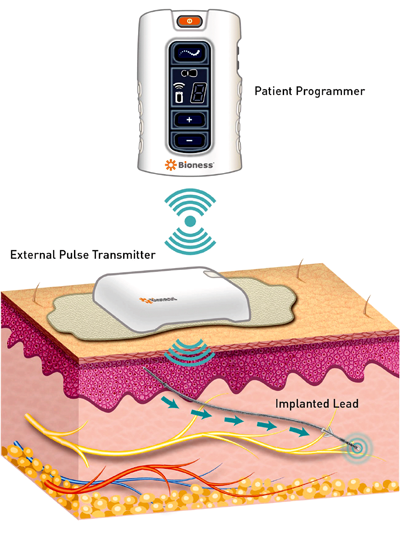 Figure 1. Implanted Bioness lead near posterior tibial nerve capturing
Figure 1. Implanted Bioness lead near posterior tibial nerve capturing
energy from the wireless external transmitter and patient programmer.
The external pulse transmitter is rechargeable and is only worn on the skin surface during periods of tibial nerve stimulation. A patient programmer is used to track usage and to change programs in the device. The programmer controls the external pulse transmitter with wireless radiofrequency. This device, which is currently being studied for OAB, has previously been tested in patients with chronic pain demonstrating no serious adverse events related to the device [3].
The second new implantable device is being developed by BlueWind Medical (Herzliya, Haifa, Israel). It is a miniature implant called RENOVA™, which is battery-less and wirelessly powered and delivers stimulation to the tibial nerve. The technology is available in two models depending on the technique used for implantation. The physician can choose between two surgical techniques, either surgical implantation or percutaneous injection based on their preference and their patient’s needs. The surgical technique consists of implanting the device through a small incision and then fixating it with a single suture in close vicinity to the tibial neurovascular bundle. The procedure can be performed under local or general anaesthesia depending on surgeon preference.
For the percutaneous technique, the device is inserted using a designated delivery system and can be positioned under ultrasound or active stimulation guidance. The device is wirelessly powered by an external control unit that controls the electro-stimulation parameters and is worn by the patient as an ankle bracelet during treatment at home. The company’s proposed treatment protocol is 30 minutes daily of self-administered treatment while carrying on normal daily activities.
Heesakkers et al. have recently published a prospective multicentre study assessing the safety and performance of the RENOVA system in the treatment of patients with OAB with or without urgency incontinence [4]. Bladder diaries were used to provide objective assessments. Clinical success was defined as one of the following outcomes: a ≥50% reduction in the number of leaks per day or number of voids per day or number of episodes with degree of urgency >2 or a return to <8 voids per day on a three-day diary. Subjective assessment was based on OAB-q including health-related quality of life (HRQL) and symptom severity score. Thirty-four of the 36 implanted subjects completed the study. At six-month follow-up, 71% experienced clinical improvement. Adverse events included: implant site pain (13.9%), suspected infection (22.2%), and procedural wound complications (8.3%).
Sacral neuromodulation (SNM)
SNM for idiopathic OAB
The goal of SNM is to modulate abnormal sensations and involuntary reflexes of the lower urinary tract restoring voluntary control of the bladder. SNM therapeutic benefits may arise from the effects of electrical stimulation on afferent and efferent nerve fibres connecting the pelvic viscera and the spinal interneurons to the central nervous system (CNS). SNM influences sacral afferents and modulates spinal cord reflexes and brain centres involved in lower urinary tract function [5].
The neurostimulator provides an electrical charge to an area near the sacral nerve, resulting in altered neural activity. This stimulation depolarises the nerve, causing an action potential. The signal propagates impulses along the axon as if the neuron had naturally fired an action potential. SNM electrically stimulates somatic afferent nerves in a sacral spinal root and sends signals to the CNS that may restore normal bladder function. Activation of somatic afferent nerves inhibits bladder sensory pathways and reflex bladder hyperactivity [6]. Unlike other therapies that target the bladder, bladder regulation occurs without physically influencing the bladder or sphincter muscles.
Interstim® (Medtronic, Minneapolis, MN, USA) has been the sole device in the market used to deliver sacral neuromodulation with demonstrable OAB therapeutic success rates as high as 83% [7]. One of the downsides of SNM therapy is device-related adverse events and surgical re-intervention.
Staged implantation of the Interstim system consists of a two-phase procedure, including a test phase in which a quadripolar electrode (tined-lead electrode) is implanted in the S3 foramen. Treatment success is usually defined by improvement of at least 50% based on bladder diaries. The test phase typically takes one to three weeks. Patients with a successful test phase proceed with the second stage, which consists of placement of the implantable pulse generator (IPG). There is also an in-office percutaneous test option where very fine wires are placed and removed after one week. If the patient has 50% improvement the entire device (lead and IPG) is then placed during one setting in the operating room.
Studies show that by 36 months of SNS therapy 11% of its subjects require battery replacement due to depletion[7]. With these results in mind, development of new SNM technologies has concentrated on providing improvements to decrease device-related adverse events and surgical re-intervention.
Axonics Modulation Technologies has recently developed a new SNM device for the treatment of OAB. The device was approved in 2016 for the treatment of OAB in Europe and Canada and consists of a small 5cm3 volume rechargeable neurostimulator (60% smaller than the currently available Medtronic SNM IPG) and a tined lead with four electrodes percutaneously inserted through the sacrum (Figure 2).

Figure 2. Size of the Axonics rechargeable neurostimulator compared to a United States quarter.
The tined lead is subsequently connected to an implantable pulse generator implanted in the upper buttock area. The implantable pulse generator battery is rechargeable with a 15-year lifetime in the body, which may obviate frequent IPG replacements. In addition, the device is current controlled, therefore, output voltage is automatically adjusted based on tissue impedance. This may provide more consistent therapy. An important consideration when implanting an SNM device is the possibility of future magnetic resonance imaging (MRI) studies for the patient. MRI studies with the Axonics SNM device are currently approved for head coil 1.5–3 T MRI’s following appropriate guidelines; at present, the company is pursuing approval for full-body MRI. This differs from the recommendation for the current Interstim-2 device, which is only approved for 1.5 T head MRI.
A prospective multicentre clinical study (RELAX-OAB trial) designed to confirm the performance of the Axonics SNM System as an aid in the treatment of the symptoms of OAB as well as capturing patient satisfaction and quality of life data was recently completed. A total of 31 of 34 patients (91%) that responded during an initial trial period (‘Test Responders’) continued to benefit from therapy with the Axonics r-SNM System at three months, defined as symptom improvement of ≥50% reduction in urinary voids or incontinence episodes or a return to <8 voids per day. No charging-related adverse events have been reported [8]. A larger study (ARTISAN) is currently in progress.
Another company, Nuvectra, is currently working on a rechargeable, current-controlled device with an option for bilateral leads. In addition, the generator for this device has the potential to provide advanced stimulation parameters and a new lead configuration which appears less likely to break if removal is needed. There are other companies currently working on the development of other sacral neuromodulation-type systems.
A rechargeable system for SNM is a welcome technological advance. However surgical revision may still be needed [9]. Therefore, while a rechargeable system would be expected to reduce costs, it will not eliminate all of the ongoing maintenance associated with neuromodulation. No matter the apparent benefits, all new technologies require continuous post-market monitoring to ensure safety and efficacy.
SNM for idiopathic non-obstructive urinary retention
Another approved indication for SNM is chronic non-obstructive urinary retention. Urinary retention without an identifiable urological cause presents a therapeutic challenge. Patients with non-obstructive chronic urinary retention usually have to rely on intermittent self-catheterisation, which has a negative impact on quality of life and may be associated with complications, such as urinary tract infections and urethral trauma. Even though its pathophysiology is not well understood, this disorder may be related to a primary failure of relaxation of the striated urethral sphincter [10].
SNM for neurogenic lower urinary tract dysfunction (NLUTD)
Recently published series on SNM for patients with neurological diseases tend to follow the same criteria used for patients with idiopathic lower urinary tract dysfunctions [11]. Indications have included refractory detrusor overactivity (DO), urinary retention (UR) due to detrusor underactivity (DU) or detrusor sphincter dyssynergia (DSD), and faecal incontinence (FI). Staged procedures were carried out, and test phase success was defined as ≥50% symptom improvement in bladder and / or bowel diaries in most studies. Positive results for selected patients were reported in a short to medium-term follow-up. Nevertheless, the US Food and Drug Administration (FDA) has not yet approved SNM as a treatment option for NLUTD. Despite this, it is commonly utilised in select neurological patients.
The current literature demonstrates positive results for selected patients with NLUTD, both for refractory urgency incontinence and urinary retention. Comparable efficacy among the neurogenic and non-neurogenic subjects have been reported in terms of success during the test phase, rates of device implantation, and clinical and urodynamic outcomes as well as quality of life. In addition, complications and reoperations are also similar in the two groups. Successful test phase rates in patients with NLUTD range from 42 to 87% and implantation success rates that range from 80 to 92%, which are comparable to implantation success rates of the non-neurogenic population [11].
“OAB is a common, disabling condition, which affects about 60 million people in the USA and neuromodulation techniques are recommended as third-line therapy – after behavioural and medical treatment has failed.”
Notwithstanding the favourable results reported on the use of SNM for patients with NLUTD, it must be emphasised that the available studies are based on small sample sizes, heterogeneous populations that are incompletely characterised in terms of neurological disease and severity of neurologic impairment, non-standardised evaluations and definitions of success, and variable follow-up. Further prospective studies with larger sample sizes, appropriate disease classification, standardised definitions of success, and longer follow-up with special attention to failure and complication rates are needed to help define the indications for SNM in patients with NLUTD [11].
One limitation with these technologies in the neurogenic population is that the current devices do not allow for MRI imaging besides head scans. While the risks are low, many radiologists may refuse to image these patients, which could present a problem if future MRIs are anticipated.
Sacral deafferentation and sacral anterior root stimulation
Sacral deafferentation and sacral anterior root stimulation by an implantable device (SDAF/SARS), often referred to as the ‘Brindley procedure’ has been developed by GS Brindley and D Sauerwein [12,13]. Basically, the procedure consists of an intradural rhizotomy of the afferent sacral nerves (usually S2–S4/5), combined with the intradural implantation of an anterior root stimulator.
The deafferentation safely abolishes detrusor activity during the filling phase, whereas the stimulator can be used for the voluntary emptying of bladder and bowels. The technique has been demonstrated to achieve safe detrusor storage pressures, voluntary voiding in physiologic intervals, and continence in patients with complete SCI, thus, resembling the normal bladder cycle more closely than any other procedure [14]. However, SDAF/SARS has never gained widespread use, even though there is evidence of clinical efficacy, positive effects on quality of life, and even cost-effectiveness [15]. This may result from the need for extra or intradural complex surgery and resection of intact nerves.
The ICS is a multidisciplinary association pertaining to the highest scientific standards and bringing a spectrum of the very best incontinence and pelvic floor disorder research from basic science to large clinical trials.
 International Continence Society
International Continence Society
TAKE HOME MESSAGE
-
New technologies for the treatment of OAB are under active development.
-
A number of implantable TNS devices are currently being studied for the treatment of OAB symptoms.
-
Interstim has been the sole device in the market used to deliver sacral neuromodulation since 1994 (approval in Europe). Therapeutic success rates have been reported to be as high as 83% in OAB patients.
-
Downsides of Interstim therapy include the need for surgical re-interventions and issues related to MRI compatibility.
-
Axonics Modulation Technologies has recently developed a new SNM device for the treatment of OAB and faecal incontinence. Other companies are currently working on other SNM devices.
-
To date, Interstim therapy is the only SNM device consistently studied, with known long-term outcomes.
-
SNM is still an off-label treatment for neurogenic lower urinary tract dysfunction. Available studies are based on small sample sizes, heterogeneous populations with non-standardised evaluations and definitions of success, and variable follow-up. Despite this, appropriate neurogenic patients can achieve successful outcomes with this technology.
References
1. Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus Sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol 2010;183(4):1438-43.
2. Peters KM, Carrico DJ, MacDiarmid SA, et al. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24-month results of the STEP study. Neurourol Urodyn 2013;32(1):24-9.
3. Deer T, Pope J, Benyamin R, et al. Prospective, multicenter, randomized, double-blinded, partial crossover study to assess the safety and efficacy of the novel neuromodulation system in the treatment of patients with chronic pain of peripheral nerve origin. Neuromodulation 2016;19(1):91-100.
4. Heesakkers JPFA, Digesu GA, van Breda J, et al. A novel leadless, miniature implantable tibial nerve neuromodulation system for the management of overactive bladder complaints. Neurourol Urodyn 2017; [Epub ahead of print].
5. Bernstein AJ, Peters KM. Expanding indications for neuromodulation. Urol Clin North Am 2005;32(1):59-63.
6. Leng WW, Morrisroe SN. Sacral nerve stimulation for the overactive bladder. Urol Clin North Am 2006;33(4):491-501.
7. Siegel S, Noblett K, Mangel J, et al. Three-year follow-up results of a prospective, multicenter study in overactive bladder subjects treated with sacral neuromodulation. Urology 2016;94:57-63.
8. Blok B, Van Kerrebroeck P, de Wachter S, et al. Three month clinical results with a rechargeable sacral neuromodulation system for the treatment of overactive bladder. Neurourol Urodyn 2018;37(S2):S9-S16.
9. Cohn JA, Kowalik CG, Kaufman MR, et al. Evaluation of the axonics modulation technologies sacral neuromodulation system for the treatment of urinary and fecal dysfunction. Expert Rev Med Devices 2017;14(1):3-14.
10. van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol 2007;178(5):2029-34.
11. Averbeck MA, Gomes CM. Worldwide utilization patterns of sacral neuromodulation for neurogenic lower urinary tract dysfunction In: Current Bladder Dysfunction Reports, Vol 11 Springer; 2016.
12. Brindley GS, Polkey CE, Rushton DN, Cardozo L. Sacral anterior root stimulators for bladder control in paraplegia: the first 50 cases. J Neurol Neurosurg Psychiatry 1986;49(10):1104-14.
13. Sauerwein D. [Surgical treatment of spastic bladder paralysis in paraplegic patients. Sacral deafferentation with implantation of a sacral anterior root stimulator]. Urologe A 1990;29(4):196-203.
14. Brindley GS. The first 500 patients with sacral anterior root stimulator implants: general description. Paraplegia 1994;32(12):795-805.
15. Wielink G, Essink-Bot ML, van Kerrebroeck PE, Rutten FF. Sacral rhizotomies and electrical bladder stimulation in spinal cord injury. 2. Cost-effectiveness and quality of life analysis. Dutch Study Group on Sacral Anterior Root Stimulation. Eur Urol 1997;31(4):441-6.
Declaration of competing interests:
Márcio A Averbeck: Medtronic (Consultant), Coloplast (Advisory Board), GSK (Consultant / Internal Expert), Ipsen (Investigator).
Howard B Goldman: Medtronic (Consultant and Study Support), Axonics (Consultant and Investigator), Allergan (Consultant), Nuvectra (Consultant), Galvani (Consultant), Bioness (Investigator), Ipsen (Investigator).









