Penile cancer is rare and accounts for less than 1% of all new cancer cases in males in the UK, with around 640 new cases diagnosed every year [1]. In England and Wales, the annual incidence is between 1.2 and 1.5 per 100,000 [2]. Traditionally, it was said a typical British urologist would see only one case a year.
As consultant numbers expand, they will see even fewer. This rarity presents several problems. The urologist will be presented with a disease he or she is not used to seeing, will probably find they are not fully conversant with the latest management strategies and will therefore find it difficult to give patients an accurate prognosis or appropriate counselling and / or reassurance. More generally, rare diseases give a paucity of material for research, hence evidence-based data is lacking in penile cancer.
However, since the National Institute for Health & Care Excellence (NICE) paper on Improving Outcomes in Urological Cancers recommended the formation of superregional networks to manage penile cancer serving catchment areas of greater than four million with the expectation that at least 25 patients were managed annually, superregional multidisciplinary teams (MDTs) have developed across the UK [3]. Although still an evidence-poor-area of urology, the management of penile cancer has evolved and certain general principles have emerged. This summary aims to provide a concise overview of penile cancer. Section headings act as statements summarising each area or principle of management.
HPV infection and chronic inflammation appear to be the two main risk factors for penile cancer.
It appears that there are two distinct penile carcinogenesis pathways in penile cancer. One is related to human papilloma virus (HPV) infection, while the other is related to chronic inflammatory processes.
Phimosis is strongly associated with penile cancer due to associated chronic inflammation [4,5]. Neonatal circumcision is associated with reduction of invasive penile cancer but does not seem to reduce the risk of the pre-cancerous penile intraepithelial neoplasia (PeIN). One of the commonest inflammatory conditions of the penis seen is lichen sclerosus et atrophicus also known as balanitis xerotica obliterans (BXO). This is commonly observed in association with penile cancer and it is thought to be a risk factor. This risk, although unknown, is believed to be low, the only series available suggesting that between 2.3% and 8.4% of BXO cases had associated or subsequently developed malignancy; however numbers in these studies were small [6].
HPV has been identified in 70-100% of PeIN cases and in 30-40% of invasive penile cancer tissue [7-9]. The HPV virus interacts with oncogenes (E6 and E7) and tumour suppressor genes (p16, P53, Rb) [7,8]. The commonest subtypes of HPV seen in penile cancer are HPV-16 and HPV-18 [10]. The other associated risk factors are smoking, poor socio-economic status and sporalene and ultraviolet A phototherapy, treatments for psoriasis.
The non-invasive precursor lesion of penile SCC is now called PeIN.
PeIN has previously been called carcinoma-in-situ (CiS), Erythroplasia de Queyrat (on the glans), Bowen’s disease (on the shaft) and has previously been designated ‘PIN’ rather than PeIN. In line with the dual pathway theory of the development of penile cancer, there are non-HPV associated precursor lesions (differentiated PeIN) and HPV associated PeIN (undifferentiated); also termed basaloid PeIN, warty PeIN, or warty-basaloid PeIN [11].
PeIN presents as red, velvety patches on the penis. It can be very difficult to differentiate from benign disease and so persistent abnormal areas should be biopsied. It is unclear what percentage of primary untreated PeIN progresses to invasive penile cancer; it has been suggested it could be up to 30% [12].
Squamous cell cancer (SCC) accounts for 95% of all penile carcinomas. Basaloid and Sarcomatoid subtypes have a poorer prognosis.
Penile cancer is most commonly a squamous cell carcinoma. The subtypes basaloid and mixed warty-basaloid (both HPV related) and sarcomatoid (non-HPV) have poorer prognosis. Verrucous and papillary (non-HPV) and warty (HPV related) subtypes, have a better prognosis. Rarer penile tumours include melanoma, sarcoma and metastatic deposits, for example from prostate or bladder malignancies.
High-grade disease, lymphovascular or perineural invasion and invasion into the corpus cavernosa have a poorer prognosis.
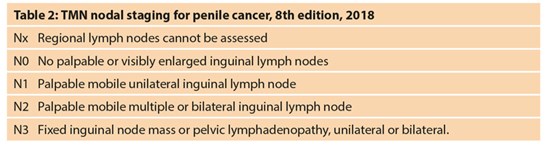
High tumour grade and lymphovascular or perineural invasion are strong predictors of poor prognosis and cancer-related mortality. Hence the latest, eighth, edition of the tumour, node, metastasis (TNM) staging classification for penile cancer is somewhat unusual as grade and lymphovascular and perineural invasion are included in the staging section. The pT1 category has been subdivided into pT1a and pT1b according to presence or absence of lymphovascular or perineural invasion or high-grade disease.
The pT2 and pT3 categories have been adapted to reflect the prognostic difference between infiltration of the corpus spongiosum (pT2) and corpus cavernosum (pT3) respectively. Disease that is able to invade the tunica of the corpus cavernosum appears to represent a more aggressive disease process with worse prognosis [13] (Table 1). Previously, TNM would classify a glans lesion with involvement of the distal urethra as T3 and therefore skew the outcome and prognosis of ‘true’ pT3 disease.
PeIN can often be managed with topical chemo- or immuno-therapy or laser ablation.
Topical agents are typically used for the treatment of PeIN. The most common agents are the antimetabolite 5-flurouracil (5-FU) or the immunomodulatory agent imiquimod. They are typically applied either once or twice a day for a period of four to six weeks [6]. They cause a significant and often frightening skin reaction and therefore patients need careful pre-treatment counselling and support during treatment to ensure compliance. This florid reaction also makes topical treatment less acceptable around the urethral meatus. Complete response rates are reported as 57% in the largest recorded series [14], although other smaller UK series have reported response rates up to 75% [15]. Close follow-up schedules are required, as failure often requires more radical treatment.
Laser ablation with carbon dioxide (CO2) or neodymium-doped yttrium aluminium garnet (Nd:YAG) lasers has been utilised for premalignant and early stage invasive lesions (Tis, Ta, and T1) with low local recurrence rates (0-6%) for premalignant lesions, (14%) for PeIN, but higher recurrence rates (22%) for T1 tumours [16].
Radiotherapy is now rarely used for primary penile cancer.
Radiotherapy is rarely used for the primary lesion of penile cancer. Local failure rates can be as high as 45% [17] and post radiotherapy changes can be functionally disabling and quite disfiguring making clinical follow-up difficult. Radiotherapy is typically reserved for patients medically unfit for surgery or as a palliative treatment. Surgical management under local anaesthesia is a preferred option in our centre in these unfit patients [18].
The aim of treatment of the primary lesion is to ensure oncological control whilst maximising penile function.
Traditional surgical management strategies for penile cancer involved surgical margins of 2cm. Several UK studies have challenged this, showing penile preserving surgery is oncologically safe with an excision margin of 5mm being adequate [19,20]. No difference has been shown between resection margins of 5 or 10mm with no local recurrences following surgery [21]. One series demonstrated a low (4%) local recurrence rate with surgical margins of only 1mm [22]. In our centre we use intraoperative frozen section in an attempt to reduce the positive surgical margin rate [23].
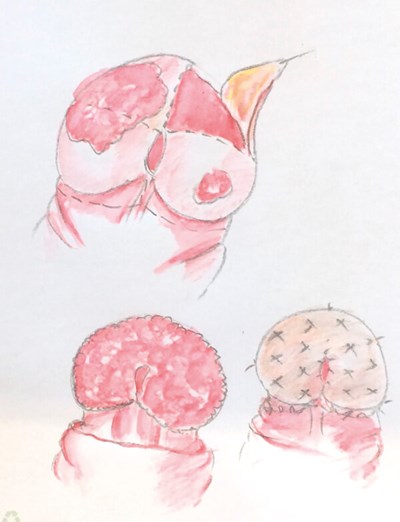
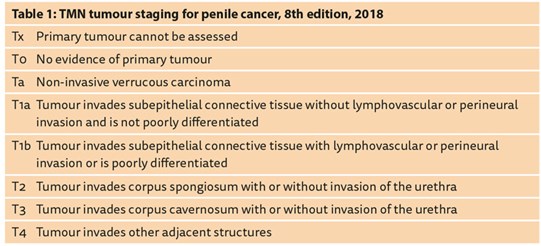
Figure 1: Glans resurfacing. The glans skin is dissected off in quadrants
removing the lesions and allowing histological analysis.
The raw surface is then covered with a split skin graft from the thigh.
Lesions that are located and confined to the prepuce can often be managed with circumcision alone, although close surveillance is required with a reported recurrence rate of up to 50% at two years [24]. Total or partial glans skin resurfacing can be offered to patients typically following relapse after topical treatments for PeIN (Figure 1). Resurfacing is also helpful if the disease involves the meatus or if further tissue is desired for histological analysis. Although there are a limited number of studies evaluating oncological outcomes for this procedure, several UK papers have shown reassuring low recurrence rates of 0-4% [25,26].
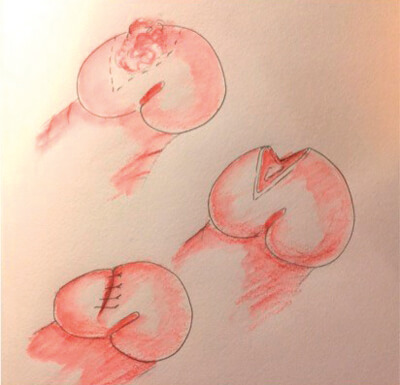
Figure 2: Partial glansectomy. The lesion is excised as a wedge from the glans;
in this case there was primary closure.
Relatively small tumours of the glans (<50% of glans) can be excised, the defect being closed primarily or reconstructed with a split skin graft; a partial glansectomy (Figure 2). Total glansectomy with or without a skin graft reconstruction is commonly utilised for larger T1 or T2 tumours (Figure 3).
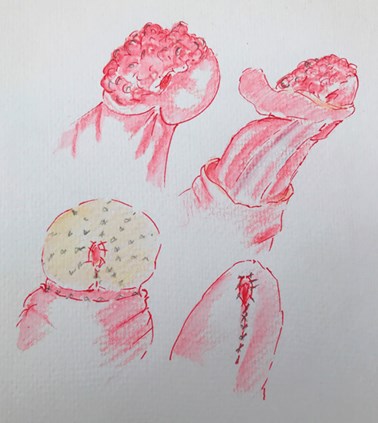
Figure 3: Glansectomy. The glans is lifted off the underlying corporal heads.
It can be reconstructed with a split skin graft or closed primarily; both are shown.
Partial penectomy remains the treatment of choice for lesions invading the corpus cavernosum (Figure 4). With distal resections and good initial penile length, split skin graft reconstruction may still be feasible.
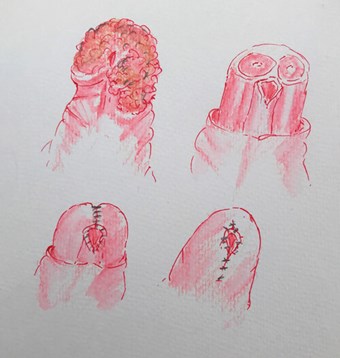
Figure 4: Partial penectomy. The disease extends into the corporal head on the left.
The corporal bodies are cut across and then closed. In this case there was primary closure.
These are examples of attempts to reduce the psychological morbidity associated with any extirpative penile surgery and aim to conserve function. It should be remembered that function does not only relate to sexual ability but also to urinary voiding. The capacity to urinate in an acceptable way (standing or sitting) is important. Partial penectomy, particularly in the overweight patient, may leave too short a penile stump, and can make voiding a messy and difficult activity. Whilst a partial penectomy may provide adequate cancer control, it is sometimes preferable to perform a radical penectomy with perineal urethrostomy for a more acceptable urinary outcome. There are of course some cases where the disease has advanced proximally to such an extent that radical penectomy is the only sensible oncological option.
The presence and extent of nodal metastases and its management remains the most important prognostic factor in survival for patients with penile cancer. Five-year cancer free survival in patients with no lymph node metastases was 92% in a UK series of penile cancer patients.
It fell to 73% in N1 disease, 61% in N2 disease and 33% in N3 disease [27] (Table 2). This is similar to other published series. Lymph node status and its management is the most important indicator of survival.
Clinically palpable or radiologically enlarged lymph nodes are malignant in 60-80% of cases [28]. This can be confirmed by aspiration cytology or excision biopsy. The management of clinically positive nodes remains radical inguinal lymph node dissection. This entails removal of all connective, fat and lymphatic tissue from around the femoral vessels in the triangle bordered by the inguinal ligament, sartorius muscle laterally and the adductor muscle medially, the two muscles crossing at the inferior apex. This extensive dissection carries a high morbidity rate of above 50%, most commonly wound infection and breakdown, seroma formation and lymphoedema [29,30]. Small case series using minimally invasive lymph node dissection with laparoscopic and robotic techniques have shown these to be oncologically safe with considerably lower morbidity and length of hospital stay and seem to support further larger-scale trials [31,32].
Patients with clinically impalpable inguinal lymph nodes remain a management conundrum. Of these patients, 20% will harbour occult metastases [33]. Prophylactic inguinal lymph node dissection in all these cases, with its high morbidity, is generally unjustified. However, a watch and wait strategy sees overall nodal recurrence in 9% of patients, compared to 2.3% who have targeted lymph node sampling by sentinel node biopsy and reduces survival from 90% to 40% if regional recurrence does occur [34]. This however includes both high and low-risk disease groups. Following primary surgery, the patient can be categorised into low (pTa/pT1 G1), intermediate (G2 pT1) or high risk (G3 & >pT1) of lymph node metastases. This allows better discussion with the low-risk patients who are offered clinical and CT surveillance and allows intermediate and high-risk groups to be offered further intervention. Generally, this intervention would be a dynamic sentinel node biopsy (DSNB).
DSNB is being adopted universally as the treatment of choice for invasive nodal staging with combined high sensitivity rates and low morbidity rates [35]. Technetium-99m (99mTc) nanocolloid and Patent Blue dye is injected around the penile cancer site or circumferentially around the penis if the lesion has already been removed and a gamma-ray probe is used intraoperatively to detect the sentinel nodes, the first in the node chain to take up the technetium and dye and thus the first to take up malignant cells. The presence of malignant cells then informs the decision to perform a subsequent lymphadenectomy of the affected side.
Patients with fixed inguinal lymph nodes (cN3) should be offered downstaging chemotherapy prior to an attempt at surgery. Radiotherapy is often also given but the evidence base is lacking. Adjuvant chemotherapy is recommended for N2 or N3 disease after lymphadenectomy [34]. The International Penile Advanced Cancer Trial (InPACT) (NCT02305654) is a large, multinational collaboration with plans to accrue 400 cN+ patients over a five-year period to be randomised into three arms: upfront inguinal lymph node dissection, neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy; the latter two followed by surgery. This trial will hopefully answer some important questions, in regards to the optimal timing of surgery and its integration with systemic therapy for nodal disease in penile cancer.
More than two malignant inguinal lymph nodes of extra-capsular spread justifies a pelvic lymphadenectomy.
More than two metastatic inguinal lymph nodes signifies a 23% risk of malignant pelvic lymph nodes; more than three and the risk increases to 56% [36,37]. There is no crossover of malignant spread to the contralateral pelvic node basin, so if more than two inguinal nodes are positive a pelvic lymphadenectomy is recommended on the same side. Extra-capsular extension of malignant cells out of a lymph node is also a high risk for further spread and so this too is an indication for pelvic lymphadenectomy. A second arm of the InPACT trial is also looking at the merits of surgery or systemic therapy for patients at high risk of developing positive pelvic nodes.
Follow-up is based on risk stratification and is for five years.
Recurrence in both the primary and nodal areas is most likely in the first two years; hence follow-up is more intensive in this initial period. After five years lymph node recurrence is rare. Local recurrence or new penile cancers may still occur, but these can be detected by the patients and so hospital follow-up ceases after five years, but self-examination is encouraged. Some (usually younger) patients may wish to have the reassurance of clinical follow-up beyond five years.
High-risk patients should be assessed every three months for two years then six months for the next three. Low-risk patients are assessed six monthly in the first two years then annually for the subsequent three. The European Association of Urology (EAU) guidelines suggest ultrasound follow-up of the lymph nodes, with fine needle aspiration if required, CT or MRI is optional. We use the latter in our centre. We also examine the chest with synchronous chest / abdomen / pelvis CT scanning in patients with sarcomatoid or basaloid disease, as they are at higher risk of distant metastases.
Diagnostic workup of the patient involves assessment of the extent of the primary lesion to plan penile surgery and of the inguinal lymph nodes for prognosis.
With all the aforementioned in mind, it becomes clearer to see the rationale for careful, targeted and speedy assessment of patients with suspected penile cancer and referral to one of the superregional centres.
Penile cancer is typically an apparent lesion but can be hidden beneath an associated phimosis. Thus, it can present as a palpable but unseen mass, discharge or bleeding and so occasionally is encountered in the haematuria clinic or during indwelling catheter changes. An urgent circumcision or dorsal slit should be arranged for these patients.
Initial examination of the penile lesion involves palpation of the penis to assess the extent of local infiltration and palpation of both groins to assess the lymph node status. Often this can only be accurately done at the time of initial biopsy under local or general anaesthesia, this may be difficult in those patients with a high BMI, but gives the first opportunity to plan subsequent surgery. A tissue diagnosis should always be obtained. However, this should not delay referral to a superregional centre.
Cross sectional imaging to include the groins and pelvis adds to the clinical examination of the nodal status. An MRI of the penis with an artificial erection may help surgical planning, but this should not delay referral and is generally carried out under the supervision of a uro-radiologist in the superregional centre.
Conclusions and the future
Significant advances have been seen for men with penile cancer. Widespread adoption of penile sparing surgery has reduced psychosexual side-effects of treatment with preservation of sexual and voiding function. Improvements in lymph node diagnostic techniques are reducing the morbidity associated with lymph node management. It is hoped the InPACT trial advances this further.
As with all healthcare issues, prevention is often better than cure. A significant impact on disease prevention may be achieved through childhood HPV vaccination of boys. The link with cervical cancer is well established with vaccination programmes introduced over the last eight years proving extremely successful [38]. The recent pathological reclassification of PeIN with regards to its association with HPV reinforces the importance of HPV pathways in penile cancer development. HPV vaccination was introduced for male children in the UK in September 2019; it will be interesting to see how this affects the incidence of penile cancer in the future.
References
1. Penile Cancer Incidence Statistics. Secondary Penile Cancer Incidence Statistics 2015.
https://www.cancerresearchuk.org/
health-professional/cancer-statistics/
statistics-by-cancer-type/penile-cancer/incidence
[accessed 15 July 2020].
2. Sewell J, Ranasinghe W, De Sylva D, et al. Trends in penile cancer: a comparative study between Australia, England and Wales, and the US. Springerplus 2015;4(1):420.
3. Kumar P, Singh S, Goddard JC, et al. The development of a supraregional network for the management of penile cancer. Annals of the Royal College of Surgeons of England 2012;94(3):204-9.
4. Maden C, Sherman KL, Beckmann AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst 1993;85(1):19-24.
5. Tsen HF, Morgenstern H, Mack T, Peters RK. Risk factors for penile cancer: results of a population-based case-control study in Los Angeles County (United States). Cancer Causes Control 2001;12(3):267-77.
6. Shabbir M, Minhas S, Muneer A. Diagnosis and management of premalignant penile lesions. Therapeutic Advances in Urology 2011;3(3):151-8.
7. Kayes O, Ahmed HU, Arya M, Minhas S. Molecular and genetic pathways in penile cancer. Lancet Oncology 2007;8(5):420-9.
8. Stankiewicz E, Kudahetti SC, Prowse DM, et al. HPV infection and immunochemical detection of cell-cycle markers in verrucous carcinoma of the penis. Modern Pathology 2009;22(9):1160-8.
9. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control 2009;20(4):449-57.
10. Muñoz N, Castellsagué X, de González AB, Gissmann L. HPV in the etiology of human cancer. Vaccine 2006;24(S 3):1-10.
11. Chaux A, Pfannl R, Lloveras B, et al. Distinctive association of p16INK4a over-expression with penile intraepithelial neoplasia (PeIN) depicting warty and/or basaloid features: A study of 141 cases evaluating a new nomenclature. American Journal of Surgical Pathology 2010;34(3):385-92.
12. Mikhail GR. Cancers, precancers, and pseudo-cancers on the male genitalia: A review of clinical appearances, histopathology, and management. J Dermatolog Surg Oncol 1980;6(12):1027-35.
13. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours, 8th Edition. Wiley-Blackwell; 2016.
14. Alnajjar HM, Lam W, Bolgeri M, et al. Treatment of carcinoma in situ of the glans penis with topical chemotherapy agents. European Urology 2012;62(5):923‑8.
15. Lucky M, Murthy KV, Rogers B, et al. The treatment of penile carcinoma in situ (CIS) within a UK supra-regional network. BJU International 2015;115(4):595-8.
16. Windahl T, Andersson SO. Combined laser treatment for penile carcinoma: results after long-term followup. The Journal of Urology 2003;169(6):2118-21.
17. Pietrzak CC, Watkin N. Organ-sparing surgery for invasive penile cancer: early follow-up data. BJU International 2004;94(9):1253-7.
18. Wardak S, Rai J, Anastasius N, et al. Glansectomy and partial penectomy for penile cancer under local anaesthesia. Journal of Sexual Medicine 2015;12(S3):249.
19. Philippou P, Shabbir M, Malone P, et al. Conservative surgery for squamous cell carcinoma of the penis: resection margins and long-term oncological control. The Journal of Urology 2012;188(3):803-8.
20. Minhas S, Kayes O, Hegarty P, et al. What surgical resection margins are required to achieve oncological control in men with primary penile cancer? BJU International 2005;96(7):1040-3.
21. Parnham AS, Albersen M, Sahdev V, et al. Glansectomy and split-thickness skin graft for penile cancer. European Urology 2016;73(2):284-9.
22. Sri D, Sujenthiran A, Lam W, et al. A study into the association between local recurrence rates and surgical resection margins in organ-sparing surgery for penile squamous cell cancer. BJUI 2018;122(4):576-82.
23. Grice P, Ellul T, Mainwairing M, et al. Long-term evaluation of local cancer recurrence rate in a large multi-centre cohort of penile cancer patients undergoing intraoperative frozen section during organ sparing surgery. Journal of Clinical Urology 2019;12(1S):6-7.
24. Li J, Zhu Y, Zhang SL, et al. Organ-sparing surgery for penile cancer: complications and outcomes. Urology 2011;78(5):1121-4.
25. Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU International 2006;98(3):532-6.
26. Shabbir M, Muneer A, Kalsi J, et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: surgical technique and outcomes. European Urology 2011;59(1):142-7.
27. Veeratterapillay R, Teo L, Asterling S, Greene D. oncologic outcomes of penile cancer treatment at a UK supraregional center. Urology 2015;85(5):1097-103.
28. Horenblas S, Van Tinteren H, Delemarre JF, et al. Squamous cell carcinoma of the penis: accuracy of tumor, nodes and metastasis classification system, and role of lymphangiography, computerized tomography scan and fine needle aspiration cytology. The Journal of Urology 1991;146(5):1279-83.
29. Stuiver MM, Djajadiningrat RS, Graafland NM, et al. Early wound complications after inguinal lymphadenectomy in penile cancer: a historical cohort study and risk-factor analysis. European Urology 2013;64(3):486-92.
30. Gopman JM, Djajadiningrat RS, Baumgarten AS, et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU International 2015;116(2):196‑201.
31. Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU International 2017;119(4):530-4.
32. Elsamra SE, Poch MA. Robotic inguinal lymphadenectomy for penile cancer: the why, how, and what. Translational Andrology and Urology 2017;6(5):826‑32.
33. Hughes B, Leijte J, Shabbir M, et al. Non-invasive and minimally invasive staging of regional lymph nodes in penile cancer. World Journal of Urology 2009;27(2):197‑203.
34. Hakenberg OW, Compérat E, Minhas S, et al. EAU Guidelines on Penile Cancer. 2020
https://uroweb.org/guideline/
penile-cancer/
[accessed 15 July 2020].
35. Zou ZJ, Liu ZH, Tang LY, et al. Radiocolloid-based dynamic sentinel lymph node biopsy in penile cancer with clinically negative inguinal lymph node: an updated systematic review and meta-analysis. International Urology and Nephrology 2016;48(12):2001-13.
36. Ornellas AA, Kinchin EW, Nóbrega BL, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. Journal of Surgical Oncology 2008;97(6):487‑95.
37. Lughezzani G, Catanzaro M, Torelli T, et al. The relationship between characteristics of inguinal lymph nodes and pelvic lymph node involvement in penile squamous cell carcinoma: a single institution experience. The Journal of Urology 2014;191(4):977-82.
38. Drolet M, Bénard É, Pérez N, Brisson M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394(10197):497-509.
Declaration of competing interests: None declared.






