Testicular tumours are the most common tumour in young males with a peak incidence seen between 25 and 34 years [1]. The overall incidence is slowly increasing, although the exact reasons for this are uncertain, and there is a greater incidence in white males compared with males of African descent. Germ cell tumour (GCT) comprise 95%, lymphoma 4% and other pathologies 1%. Lymphoma is generally classed as a separate entity as the treatment is different, and this is more commonly seen in older patients.
The prognosis is generally very good, even in patients with metastatic disease at the time of diagnosis. Overall five-year survival is 99.2% in patients with localised disease, 96.1% in patients with spread to regional lymph nodes and 73.2% for patients with metastatic disease [1].
Risk factors
Risk factors include previous testicular malignancy, family history (four times increase if father had testicular malignancy and nine times increase if brother had testicular malignancy), subfertility, undescended testis (even following orchidopexy). Previous studies suggest that 5-20% of patients with a history of undescended testis will develop a tumour in the contralateral descended testis [2]. Microlithiasis was previously considered as a possible risk factor, but recent guidelines state that this only needs radiological surveillance if another independent risk factor is present and that microlithiasis in itself is not a risk factor.
“MRI is not used routinely in staging, but can be used for problem solving of an unusual or indeterminate testicular lesion.”
Ultrasound
Ultrasound (US) is the recommended first-line investigation in a patient with a suspected testicular tumour or palpable mass. Most palpable lumps felt by the patient are likely to be benign, due to other pathologies such as epididymal cysts, scrotal calcification, or testicular cysts. Ultrasound will confirm the presence of a testicular mass and it is important to evaluate the mass, looking for areas of calcification or fluid and at the overall vascularity. It is really difficult to give a pathological type of tumour based on ultrasound alone as the appearances can have similar characteristics.
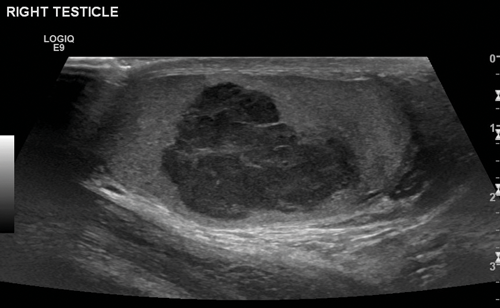
Figure 1. US of seminoma seen as a multi-septated area of low attenuation with a lobulated outline.
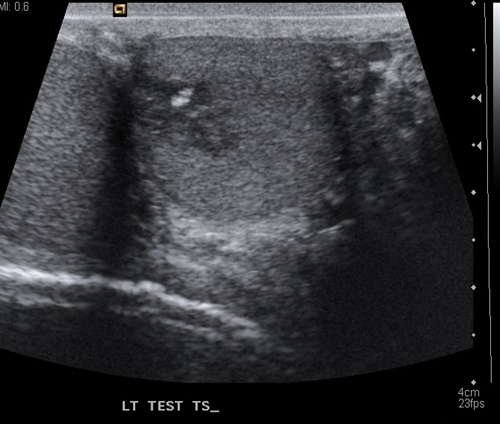
Figure 2.US of teratoma seen as a low echo lesion containing an area of central calcification.
Seminomas can have a uniform appearance and are generally of low echogenicity but larger tumours may be heterogenous and lobulated (Figure 1). Typically, teratomas are described with areas of calcification and are heterogenous, also containing fluid. They can have areas of central necrosis and haemorrhage (Figure 2). Both tumours are classically hypervascular, but depending on areas of fluid or necrosis may be hypovascular. Lymphoma tends to be infiltrative involving the whole testis and causing diffuse testicular enlargement rather than a more focal tumour.
Staging
Staging CT is usually performed following orchidectomy as the primary tumour should be removed as soon as possible. The Royal College of Radiologists recommends CT chest, abdomen and pelvis for complete staging, looking for lymph nodes and distant metastases, which are most commonly seen in the lung, liver and brain. The primary tumour is not assessed on CT or MRI, and as the patient is usually postoperative, changes may be seen in the inguinal region from the recent surgery.
Most patients are diagnosed at an early stage and have an excellent prognosis. Seventy-seven percent of patients have stage one disease at diagnosis and 14% stage two with only 9% having a higher stage and 1% having distant metastases at the time of diagnosis.
Stage one disease is where there is no nodal or metastatic disease and, apart from incidental findings, the staging CT is essentially normal.
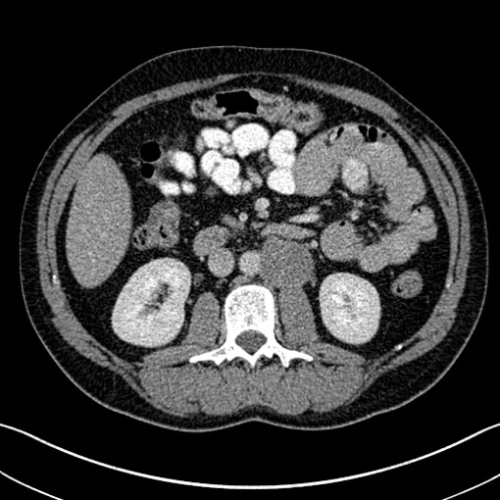
Figure 3. Axial CT showing left para-aortic lymph node from a left sided tumour.
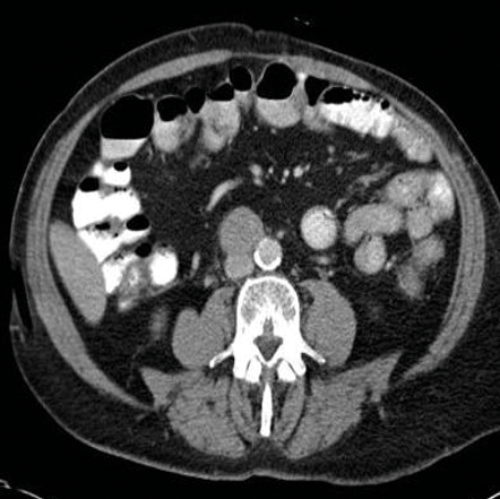
Figure 4. Axial CT showing aortocaval lymph node from a right sided tumour.
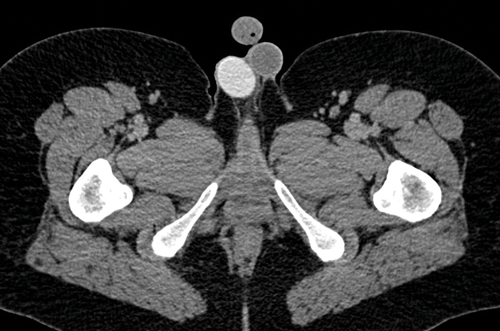
Stage two disease has abdominal lymph nodes; 2a nodes <2cm, 2b nodes 2-5cm, 2c at least one node >5cm. Retroperitoneal lymph nodes are the commonest site for metastatic disease and left sided tumours tend to spread to left para-aortic lymph nodes (Figure 3) and right sided tumours to aorto-caval or precaval nodes [3] (Figure 4). It is important to carefully evaluate the relevant area depending on the site of the tumour.
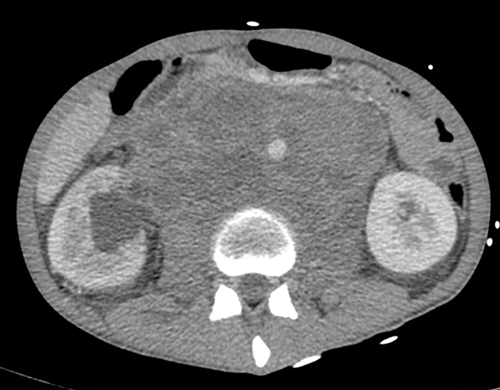
Figure 5. Massive retroperitoneal lymphadenopathy causing effacement of
the IVC, anterior displacement of the aorta and right hydronephrosis.
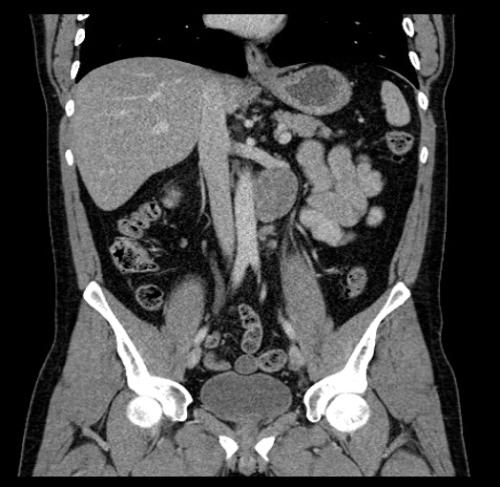
Figure 6. Coronal CT showing left para-aortic lymph node in the same patient as Figure 3.
Nodes may be massive and encase renal vessels, displacing the aorta or inferior vena cava (IVC) and this is important when reporting the staging CT (Figure 5). Coronal views can be very useful to demonstrate the overall length of nodal disease as sometimes, on axial imaging the size of the nodes can be underestimated (Figure 6). Teratoma nodes may contain some areas of calcification or low attenuation areas of necrosis, which are less often seen in seminoma lymph nodes. Retroperitoneal lymph nodes often respond very well to chemotherapy agents, but teratoma nodes may require retroperitoneal lymph node dissection if they do not respond or there remains a sizable node at the end of the treatment.
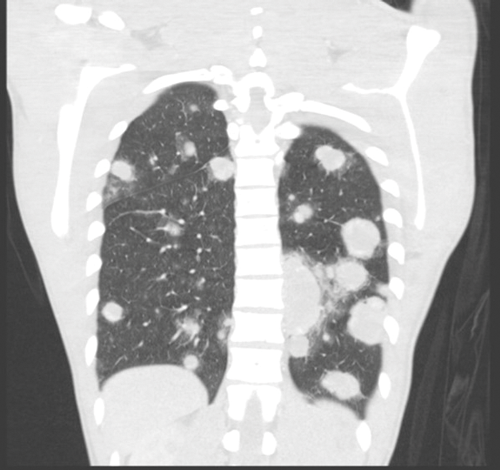
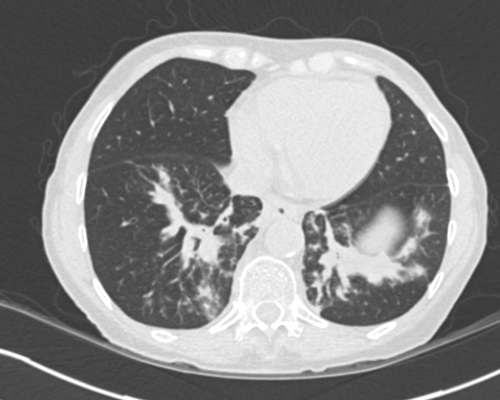
Figure 7. Bilateral lung metastases seen on coronal CT.
Stage three is where there is spread to distant nodes or other organs, most commonly the lungs and the liver (Figure 7).
Brain imaging is advised if there are over 20 lung metastases, HCG is >10,000 or there is focal neurology. The brain metastases are often haemorrhagic so are high attenuation on pre-contrast CT [4].
MRI
MRI is not used routinely in staging, but can be used for problem solving of an unusual or indeterminate testicular lesion, particularly in cases where orchidectomy is not optimal such as patients with previous orchidectomy. MRI can help evaluate a testicular lesion and can demonstrate adjacent anatomy clearly. A solid testicular tumour generally has lower signal on T2-weighted MRI than the usually seen high signal of the normal testis [5].
Figure 8. Axial CT shows bilateral lower lobe linear opacities with thickening
of the bronchovascular markings in keeping with bleomycin lung.
Complications of treatment
Bleomycin lung can be seen in some patients, and is typically seen as linear areas of atelectasis in the lower lobes (Figure 8). It can be subtle or more florid, and if patients are still taking bleomycin, this is stopped and they may be treated with steroids.
The radiological appearances may not improve after treatment and it is an area that the radiologist should be aware of when reporting post-treatment CT.
Figure 9. Axial CT shows a right sided testicular prosthesis which is of high attenuation due to the internal contents.
Testicular prosthesis
Testicular prosthesis can be inserted at the time of initial surgery, at a later date, or patients may decide not to have a prosthesis. The prosthesis can be seen on subsequent CT and is usually well defined and of high attenuation, due to the internal contents (Figure 9). A prosthesis on ultrasound has a very echogenic anterior border but dense posterior acoustic shadowing, and nothing can be seen behind the anterior border.
“Testicular prosthesis can be inserted at the time of initial surgery, at a later date, or patients may decide not to have a prosthesis.”
Conclusion
Ultrasound is useful to identify a testicular tumour, but not for histological classification. CT should be performed for staging purposes, and carefully evaluated in the left para-aortic area for left sided tumours and the aorta-caval region for right sided tumours. Any encasement or displacement of vessels should be clearly reported. MRI can be useful as a problem-solving tool, but is not routinely used for either imaging of the testis or staging of testicular malignancy.
References
1. Surveillance, Epidemiology and End Results Program. SEER Stat Fact Sheets: Testicular Cancer. National Cancer Institute. Available at
http://seer.cancer.gov/
statfacts/html/testos.html
2. Petterson A, Richiardi L, Nordenskjold A, et al. Age at surgery for undescended testis and risk of testicular cancer. N Engl J Med 2007;356(18):1835-41.
3. Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol 1982;128:315-20.
4. Oechsle K, Bokemeyer C. Treatment of brain metastases from germ cell tumors. Hematol Oncol Clin North Am 2011;25:605-13
5. Kreydin EI, Barrisford GW, Feldman AS, Preston MA. Testicular cancer: What the radiologist needs to know. American Journal of Roentgenology 2013;200(6):1215-25.