In the second part of our comprehensive overview of urinary incontinence (UI) the authors explore the plethora of treatment options for this complex condition. (Part 1 available here).
Conservative management
Initial treatment of incontinence should be conservative. Caffeine reduction and bladder retraining can be effective for overactive bladder (OAB) but not necessarily urgency urinary incontinence (UUI) [1]. The patient is asked to hold on to progressively larger bladder volumes to encourage the bladder capacity to increase with suppression of urgency. Pelvic floor muscle training (PFMT), supervised by a trained continence advisor or physiotherapist, can be effective for both stress urinary incontinence (SUI) (reducing hypermobility) and less so UUI [2]. In obese patients there is a four-fold increase in the likelihood of UI; weight loss of only 5% in the obese patient can improve UI severity and quality of life (QoL) [3].
SUI can be managed by urethral inserts or vaginal pessaries, which aim to occlude the urethra. These have a specific role in clinical practice, such as in younger women who require temporary protection during sport or would prefer to defer SUI surgery until after completion of their family, or in older women who may be unfit for surgery.
All patients should be offered help with containment of their incontinence while treatment is initiated. Pads are frequently used, but catheter placement is sometimes necessary in the elderly where reversible causes have been exhausted and perhaps where dementia or impaired mobility prevents correction of their continence status.
Medical management for UI
Topical vaginal oestrogens have been shown to be effective in treating OAB and UUI in post-menopausal women [4]; they are thought to improve vaginal tone and periurethral support. There is no evidence for systemic oestrogens in UI and they can cause worsening of SUI.
Antimuscarinic agents (e.g. oxybutynin, tolterodine, fesoterodine, solifenacin, darifenacin and trospium) are commonly used for OAB and UUI [5]. Although effective to a degree, only a small proportion remain on them long-term consequent to adverse effects or inadequate response to the drugs. Dry mouth and constipation are common, and worsened confusion in the elderly can be problematic. However, there is emerging data on the safe use of some of these in the elderly.
Mirabegron is a Beta-3 adrenergic agonist, which was licensed for treatment of OAB / UUI in 2013. It has similar efficacy to anticholinergics but without the above side-effects [6]. Its place in the treatment algorithm is yet undecided but it may be used first-line particularly where antimuscarinics are contraindicated, such as in narrow angle glaucoma, or where antimuscarinics are likely to be poorly tolerated, such as in the elderly.
Anticholinergics and mirabegron are ineffective for SUI. The only medication licensed for SUI treatment is duloxetine, a selective serotonin re-uptake inhibitor (SSRI). However, due to its limited efficacy and significant side-effects (predominantly nausea, vomiting and dizziness), it is only recommended for patients who are unsuitable for, or have declined, surgical intervention.
Surgical treatment for UI
If conservative measures fail to improve UI sufficiently, surgery may be required. The patient is fully assessed and counselled regarding the surgery and its potential complications. This includes take-home literature. The need to be able to perform intermittent self-catheterisation (ISC) should be discussed as a potential risk. It is usual practice to address the UI with the greatest degree of bother first. Often other components of UI do not require treatment due to the overall improvement in QoL when the most bothersome component has been dealt with.
Surgery for SUI
When considering surgery for SUI the factors the clinician has to consider are:
- Is this urethral hypermobility or intrinsic sphincter deficiency (ISD) or both?
- Is there coexistant detrusor overactivity (DOA) or impaired detrusor contractility?
- Is there concomitant vaginal wall prolapse?
Surgery can be classified as those that provide a peri-urethral support for urethral hypermobility and those to support urethral closure in ISD. The supportive procedures provide a backboard to prevent urethral hypermobility. These can be again divided into suspension vs. backboard procedures.
Suspension procedures
The Burch Coloposuspension (and its variations) is not often performed anymore but deserves a mention as it remains a useful option. It was designed to attach the peri-urethral tissues to a fixed lateral structure to prevent descent. The surgery requires adequate vaginal length and mobility. In the usual technique the peri-urethral supportive tissues are approximated to the ileopectineal line (ligament of Cooper) employing two to four absorbable (poldiaxanone) sutures used on each side. It is important not to over-tighten the sutures leading to retention or use non-absorbable sutures as this can cause erosion. Open Burch has a long-term efficacy of 85%. The procedure can also be performed via laparoscopic or robotic techniques [7]. Complications of the colposupension include haematuria, de-novo instability (30%), retention of urine (10%, of which 5% resolve), vaginal prolapse (20% - usually asymptomatic) and post colposuspension syndrome (10%).
Backboard procedures
In these procedures firm support is created behind the posterior urethra to prevent descent and allow closure of the urethra during episodes of raised intra-abdominal pressure. Mid-urethral slings (e.g. tension free transvaginal tape – TVT, transobturator tape – TOT) are the commonest operations for stress incontinence. A type 1 monofilament macroporous mesh tape is placed under the mid-urethra, which provides supportive tissue behind the posterior urethra to prevent descent and allows the passage of fibroblasts, collage and blood vessels (Figure 1).
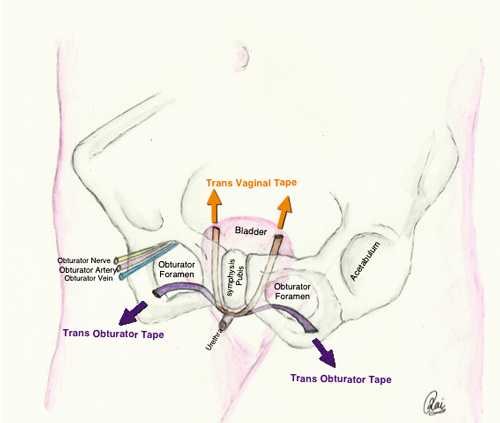
Figure 1.
The efficacy is good (around 85% cured or improved), and the procedure is well tolerated [8] although associated with significant, albeit uncommon, morbidities and risks. Some patients can develop difficulties emptying the bladder requiring clean intermittent self catheterisation (CISC), de-novo urgency, or local discomfort / thigh pain (TOT perforation of adductor longus tendon). Rarely, the mesh can extrude through the tissues into the vagina, urethra or bladder. In the instance of erosion the extruded mesh has to be removed.
Where synthetic mesh is to be avoided, a similar procedure can be performed using natural tissue such as rectus sheath or fascia lata. The procedure is more invasive as it requires graft harvesting, but useful in failed SUI surgery, in neuropathic patients with SUI and other indications [7].
Figure 2.
Augmentation of urethral closure procedures
In the presence of pure ISD with no hypermobility, bulking agents, such as polydimethylsiloxane (Macroplastique®), Hyaluronic acid / dextran co-polymer (Deflux®), collagen or zirconium beads, can be injected around the urethra using a cystoscope to promote coaption of the mucosa and thereby reduce stress leakage. The efficacy is relatively poor (<50% cure rates long-term) although better than placebo, but the side-effects of retention and de novo OAD are transient [9]. The procedure can be performed via local anaesthetic flexible cystoscopy. The procedure is often required to be repeated to achieve improved efficacy.
Pubovaginal slings
The indications for slings include previous failed mid-urethral tapes, neurogenic bladder dysfunction, erosion, fistula or tissue loss (where mesh should be avoided) and in patients who require to perform CISC long term. The patient requires a compliant bladder.
Different types of sling materials are used:
- Autograft: autologous rectus fascia, fascia lata. Rectus fascia is better than fascia lata as it avoids leg symptoms.
- Allograft: cadaveric dura mater, cadaveric fascia lata, acellular dermis. Sterilisation of the allograft by solvents and freeze drying but risk of HIV (1 in 8 million) and CJD (1 in 3.5 million).
- Xenograft: porcine small bowel submucosa which has less immunological response than bovine pericardium.
- Synthetic sling.
Autologous sling achieves a higher rate of success than the Burch colposuspension. However it also has a greater morbidity [7].
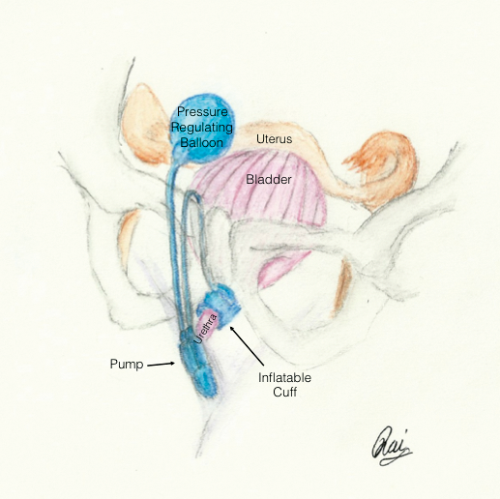
AUS
An artificial urinary sphincter (AUS) is indicated for type 3 SUI or ISD. The AUS is composed of three parts (Figure 2). The cuff that sits around the urethra and when filled compresses the urethra to prevent UI. The cuff is connected to a small pump that sits in the labia and when manually activated pumps fluid into the reservoir that is sited in the abdomen. Once fluid leaves the cuff, the urethra is uncompressed and voiding can occur. The reservoir will passively refill the cuff to restore continence over a two-minute period. There is a high cure rate of >90% but a high erosion rate (7-29%); consequently this procedure is often reserved for individuals who have failed previously SUI surgery with a fixed scarred urethra [10].
In a failed mid-urethral tape, a further option is to insert a second tape leaving the first in situ. Here, there would be equivalent efficacy and similar complications to the primary procedure. The alternative is to perform either a Burch colposuspension or autologous fascial sling.
Surgery for UUI
Intravesical injections of botulinum toxin (e.g. Botox) can be highly effective for the treatment of OAB and UUI. The success rate is reported as between 70% and 85% [11]. The treatment lasts for around 6-12 months, after which further injections can be administered. Around 5-15% of patients cannot adequately empty their bladder after treatment, and may need to perform CISC for several weeks to months. Botox selectively cleaves the SNAP-25; which prevents normal vesicle docking and fusion to the presynaptic plasma membrane. It inhibits the release of acetylcholine but not its production or destruction. It reduces the level of sensory receptors (TRPV1 and P2X3) in the suburothelium which results in decreased sensitivity of the afferent unmyelinated C fibres [12]. The motor effects of Botox last less then the sensory effects and patients who perform CISC secondary to Botox often have normal voiding restored before the symptoms of OAB return.
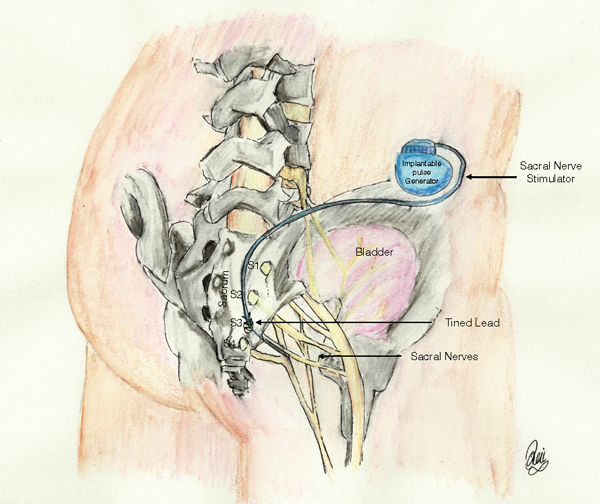
Figure 3.
Other treatments for UUI include neuromodulation [13]. This involves the use of direct electrical stimulation of nerves of S3 (Figure 3). This is either through an implanted stimulator (sacral nerve stimulation, SNS) or a transcutaneous approach (percutaneous tibial nerve stimulation – PTNS).
“The condition may be difficult to assess and manage, consequent to the complexity of the underlying causative factors, combined with our currently incomplete understanding of the lower urinary tract control mechanisms.”
SNS is a National institute for Health & Care Excellence (NICE) approved technique that requires a short (four to six week) trial with temporary leads placed in the S3 foramen and an externally worn battery and control device. This is programmed with different settings and the patient assessed before and after the trial. If there is improvement the patient has a permanent tined lead inserted into the S3 foramen and subdermal tunneling of the lead to a battery-powered controller that is buried in either the lower flank or above the belt line in an area marked preoperatively. This can be controlled externally via radio control but the battery requires changing every five years. The battery life is also dependent on the settings for the patient. Those using a higher energy for effect will require more frequent battery change. The closer the lead placement to the S3 nerve the lower the voltage used to achieve effect and the longer the battery life. The patient has to be made aware that once sited it is no longer possible to have an MRI scan or use monopolar diathermy. The implants are expensive and the capital costs are high.
PTNS is a minimally invasive outpatient-based procedure. PTNS is limited by the need for weekly sessions. SNS and PTNS are expensive options but have few side-effects and no incidence of incontinence. They are also used for pelvic floor disorders, faecal incontinence and pelvic pain. SNS has been used as a treatment of retention from Fowlers syndrome. They offer up to 80% improvement in UUI [14].
Reconstructive surgery
Reconstructive surgery, such as augmentation cystoplasty (e.g. ileocystoplasty), involves bi-valving the bladder and a section of detubularised small bowel is sutured to the gap to expand bladder capacity. Although it has a success rate of approximately 80%, this is a major surgical procedure with long-term morbidity. The patient should be able and willing to perform ISC long term, and problems with mucous collection, bladder stones and recurrent infections are not uncommon. Augmentations are uncommonly performed now, consequent to the increased use of less invasive options such as Botox and SNS.
Urinary diversion
For patients unresponsive to the above mentioned treatments, urinary diversion offers a final procedure to treat UI. A section of ileum is isolated and used to join the ureter to the abdominal wall (i.e. a stoma). The bladder can either be removed or left in place. Although the original incontinence is certain to be cured in 50-70%, stomas can be complicated by stenosis, hernia, metabolic disturbances including renal failure in up to 30%, recurrent urinary infections, stones and pyocystis in the original bladder and a small risk of malignancy. This is extensive surgery with added morbidity with a cystectomy. There may be psychological problems and body image issues associated with a stoma. Urine may also still leak due to poorly fitting stoma devices, particularly if the body habitus is not optimal.
Conclusion
Urinary incontinence in women is diagnosed simply by their complaints of urinary leakage. However, the condition may be difficult to assess and manage, consequent to the complexity of the underlying causative factors, combined with our currently incomplete understanding of the lower urinary tract control mechanisms. There is considerable overlap between findings in asymptomatic and symptomatic women, and symptoms may not correlate with signs or investigation findings. There are a plethora of treatment options as we have described above, but no ‘magic bullet’. Management options and the approach to evaluation will vary between the age groups as well as the underlying pathologies, as geriatric assessment will be very different from that in a young woman, and a neuropathic bladder requires a different approach. All the options of management may have serious adverse consequences and should be discussed with patients in detail before embarking on surgery. Urinary incontinence is a complex topic, which requires careful and detailed assessment.
References
1. Townsend MK, Resnick NM, Grodstein F. Caffeine intake and risk of urinary incontinence progression among women. Obstet Gynecol 2012;119(5):950-7.
2. Hay-Smith EJ, Herderschee R, Dumoulin C, Herbison GP. Comparisons of approaches to pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst Rev 2011;(12):CD009508.
3. Auwad W, Steggles P, Bombieri L, et al. Moderate weight loss in obese women with urinary incontinence: a prospective longitudinal study. Int Urogynecol J Pelvic Floor Dysfunct 2008;19(9):1251-9.
4. Cody JD, Jacobs ML, Richardson K, et al. Oestrogen therapy for urinary incontinence in post-menopausal women. Cochrane Database Syst Rev 2012;10:CD001405.
5. Chapple CR, Khullar V, Gabriel Z, et al. The effects of antimuscarinic treatments in overactive bladder: an update of a systematic review and meta-analysis. Eur Urol 2008;54(3):543-62.
6. Chapple CR, Kaplan SA, Mitcheson D, et al. Mirabegron 50 mg once-daily for the treatment of symptoms of overactive bladder: an overview of efficacy and tolerability over 12 weeks and 1 year. International Journal of Urology 2014;21(10):960-7.
7. Albo ME, Richter HE, Brubaker L, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med 2007;356(21):2143-55.
8. Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. The Journal of Urology 2015;193(1):203-10.
9. Ghoniem GM, Miller CJ. A systematic review and meta-analysis of Macroplastique for treating female stress urinary incontinence. International Urogynecology Journal 2013;24(1):27-36.
10 Kowalczyk JJ, Mulcahy JJ. Use of the artificial urinary sphincter in women. Int Urogynecol J Pelvic Floor Dysfunct 2000;11(3):176-9.
11. Tincello DG, Kenyon S, Abrams KR, et al. Botulinum toxin a versus placebo for refractory detrusor overactivity in women: a randomised blinded placebo-controlled trial of 240 women (the RELAX study). Eur Urol 2012;62(3):507-14.
12. Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol 2006;49(4):644-50.
13. Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. The Journal of Urology 2006;175(3 Pt 1):835-41.
14. Noblett K, Siegel S, Mangel J, et al. Results of a prospective, multicenter study evaluating quality of life, safety, and efficacy of sacral neuromodulation at twelve months in subjects with symptoms of overactive bladder. Neurourology and Urodynamics 2014 [Epub ahead of print].
Declaration of competing interests: None declared.