Renal angiomyolipoma (AML) are benign tumours, accounting for approximately 2–3% of all renal neoplasms [1]. Seventy percent of renal AMLs are sporadic, and 20–30% are associated with genetic aetiology. They are composed of smooth muscle, blood vessels, and adipose tissue.
With the increased availability of imaging, most AMLs are initially detected on ultrasound scans either directly or identified as renal masses, prompting referral to a urologist for further evaluation. This contrasts with previous decades when AMLs were typically diagnosed during evaluation for symptoms or complications rather than being incidentally discovered.
Although asymptomatic presentation as an incidental finding is the most common, the likelihood of symptoms and complications increases with the size of the AML. The most frequent symptoms in decreasing frequency, are flank pain, haematuria, nausea and vomiting, and abdominal distention [2]. These symptoms and complications are more prevalent in females of reproductive age, as the tumour strongly expresses oestrogen and progesterone receptors [3]. Clinical features also depend on the aetiology of the AML. Sporadic AMLs typically occur in the fourth and fifth decades, presenting as incidental, solitary masses with slow growth. In contrast, genetic AMLs, more commonly seen in association with tuberous sclerosis and less so with lymphangioleiomyomatosis, tend to occur at a younger age, often in the second and third decades of life, and present as bilateral, multiple, and rapidly growing symptomatic lesions. Epithelioid AML is a rare, aggressive variant with potential for distant metastasis at diagnosis.
Despite their benign nature, one of the life-threatening complications of AML is retroperitoneal bleeding, also known as Wunderlich syndrome. The risk factors for bleeding include female sex, reproductive age, larger tumour size, and genetic associations.
This article primarily focuses on the imaging modalities and surveillance strategies for sporadic renal AML. Active treatment of AML is a different topic and is outside the scope of current discussion.
Imaging in renal AMLs and different modalities
As renal AML is increasingly being diagnosed incidentally, it is crucial to obtain a definitive diagnosis through imaging and avoid invasive investigations unless necessary. Many patients are referred to a urologist with an ultrasound report of an incidental finding of a renal mass or AML.
Ultrasound
Although ultrasound is generally less sensitive and specific for detecting renal masses smaller than 2cm, AMLs are an exception due to their distinct features. Fat is hyperechoic on ultrasound, making AML typically appear as hyperechoic masses with posterior acoustic shadowing. While a renal cell carcinoma (RCC) may also appear echogenic, AMLs are usually more echogenic than the renal sinus. In contrast, RCCs are typically less echogenic than the renal sinus and are surrounded by a rim of hypoechoic tissue, likely due to the tumour’s compression of normal kidney tissue [4]. Besides assessing the vascularity, Doppler ultrasound is not significant in diagnosing AML. There is a diagnostic overlap in fat-poor or fat-invisible AML or in an RCC that may rarely contain fat, which makes differentiation challenging. Due to reduced fat content, fat-poor AML will appear relatively isoechoic to the surrounding kidney tissue. In such cases, cross-sectional imaging becomes indispensable.

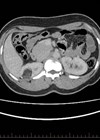
CT scan showing a left renal angiomyolipoma measuring 3cm (arrow).
CT
Fat has low attenuation on CT scan and appears dark. On CT, the hallmark feature of AML is the presence of fat, seen as areas of decreased attenuation distinct from the surrounding renal tissue. This makes diagnosing fat-rich AML straightforward [5]. RCC may demonstrate irregular margins, calcifications, invasion into surrounding structures, or renal vein thrombus – findings never seen in AMLs. Fat-invisible AMLs are particularly difficult to diagnose on CT, as they appear hyper attenuating without the typical fat-related characteristics and may require additional diagnostic modalities, such as MRI.
While contrast enhancement has a limited role in directly diagnosing AMLs, it provides valuable information about vascularity, the presence of aneurysmal vessels, and the potential for retroperitoneal haemorrhage as the aneurysms larger than 5mm are at an increased rupture risk.
When should MRI be preferred over CT in imaging AMLs?
MRI is pivotal in differentiating fat-poor and fat-invisible AMLs from RCC. T2-weighted sequences and diffusion-weighted imaging (DWI) are vital tools. Differentiating fat-poor AMLs from clear cell RCC is challenging because fat-poor AMLs contain minimal scattered fat, while clear cell RCCs have intracytoplasmic lipids within tumour cells, leading to similar characteristics on CT scan [6]. Similarly, differentiating fat-invisible AMLs from non-clear cell RCC is challenging because fat-invisible AMLs lack fat. Non-clear cell RCC contains little to no intracytoplasmic lipids, making them appear similar on CT imaging [6] (for further information refer to Table 1).

On T2-weighted images, fat-rich AMLs appear hyperintense due to their fatty tissue, while fat-poor AMLs are hypo intense compared to the renal parenchyma. It is even more challenging to differentiate fat-invisible AML from RCC as they completely lack adipose tissue and resemble renal parenchyma. Clear-cell RCC is hyper intense on T2, while fat-poor AMLs are hypo intense. However, both fat-invisible AMLs and non-clear-cell RCCs are homogeneously hypointense on T2-weighted images.
On DWI, signal intensity is inversely proportional to fat content. Fat-poor AMLs show restricted diffusion and appear hyper intense on DWI, whereas clear-cell RCCs are hypointense. Fat-invisible AMLs and non-clear-cell RCCs are both homogeneously hypointense on T2 and exhibit strong diffusion restriction on DWI, further complicating differentiation.
One of the most significant challenges remains distinguishing fat-invisible AML from non-clear-cell RCC, as both present similarly on T2-weighted and DWI images, with high signal intensity due to diffusion restriction.
When should a biopsy be considered for AML?
In fat-rich AML, biopsy is rarely needed as they are easily diagnosed on CT. In fat-rich AML, the main indication for biopsy would be the presence of intra-tumoral calcification or necrosis to rule out a fat-containing RCC, which is rare. In fat-poor AML, the role of biopsy is controversial since they can often be differentiated from RCC using MRI, specifically with T2-weighted images and DWI [7]. Biopsy can help distinguish between fat-invisible AML and non-clear cell RCC, as they are challenging to differentiate on CT and MRI [7]. Despite being an efficient diagnostic tool, a notable limitation of biopsy is its non-diagnostic yield of around 20%.
Technetium-99m sestamibi scan: an emerging tool in the diagnosis of AML
Tc 99 sestamibi (MIBI) single photon emission computed tomography (SPECT) / CT is an evolving investigation to differentiate solid benign renal tumours from RCC. The principle involved in the MIBI scan is based on the mitochondrial activity of tumour cells. Benign tumours have increased mitochondrial density, leading to an increased tracer uptake. In contrast, malignant tumours have low mitochondrial density and increased multidrug-resistant pumps, which efflux Tc 99, resulting in decreased tracer uptake [8]. The sensitivity of the MIBI scan is 100%, and the specificity ranges from 95% to 96% for distinguishing benign renal lesions from renal cell carcinoma [9]. Although the technetium-99m sestamibi scan is not routinely recommended for the diagnosis of AML due to limited supporting evidence, it is undoubtedly an evolving and valuable tool for assessing indeterminate renal lesions and avoiding biopsies.
Surveillance of sporadic AML and the imaging modality of choice
Given AMLs benign and often asymptomatic nature, surveillance is a safe and reasonable approach. Studies have shown that during active surveillance, the rate of growth in AML is generally slow, with only 11% of patients experiencing growth [10]. Spontaneous rupture leading to bleeding or haematuria occurs in around 2% of cases. The likelihood of requiring active treatment later is relatively low, at approximately 6% [10]. Symptomatic lesions (e.g. pain, haematuria) often require active management (embolisation / surgery).
The surveillance mainly depends on non-modifiable factors such as tumour size, growth rate, genetic predisposition, and patient-specific characteristics like age and gender. It should also be tailored according to comorbidities and social factors.
The decision to monitor or treat AML depends on a variety of factors, which are outlined in Table 2.

As the size of tumour increases, so does the risk of bleeding and symptoms. Most studies state that AML less than 4cm can be actively surveyed unless symptomatic. However, this previous 4cm cut-off has recently been challenged as a risk factor for bleeding, as it has led to the overtreatment of many asymptomatic AMLs. Some studies have concluded that the increased risk of bleeding is more pronounced in lesions exceeding 6cm rather than 4cm [9]. Larger tumours could also be considered for AS in cases of severe patient comorbidity, solitary kidney, or the presence of Bosniak IIF cysts.
Recently, there have been changes in the surveillance of renal AML with evidence suggesting that asymptomatic tumours less than 2cm do not require follow-up. While size was previously the primary determinant of treatment, recent findings highlight the importance of individualised risk assessment, tumour growth rate, and vascularity. The final decision to actively survey or treat should ideally be based on a balanced approach to avoid bleeding or other complications while avoiding over-treatment.
According to Chan et al., most sporadic AMLs, particularly those under 40mm, do not grow significantly and rarely pose a threat. Their study recommends different surveillance strategies based on tumour size. Their practice was to perform a US scan five-yearly for AMLs 21–29mm and a two-yearly US for 30–39mm tumours [12]. Maclean et al. examined the growth rate of small solitary AMLs under 20mm and concluded that this subgroup do not require follow-up due to their slow growth rate [13]. Ouzaid et al. mentioned that their AS protocol consisted of imaging at 6–12-month intervals, followed by annual follow-up visits, including a physical examination and CT imaging [14]. Dorin et al. reported a retrospective case series where patient follow-ups occurred at 6–12-month intervals using CT, MRI, or US. AMLs <4cm in diameter were considered for annual surveillance [15].

Proposed guidelines for surveillance of sporadic AML. CT is ideal for surveillance as it can clearly diagnose most AMLs and provide a better assessment of tumour size, fat content and risk of bleeding. However, it carries the concern of radiation exposure. MRI, conversely, can be used for AMLs with less to no fat, it avoids radiation but is more costly and less readily available than CT. In small slowly growing AMLs US may also be used for surveillance provided a baseline US study is available for comparison.
Conclusion
Although several studies have reported on surveillance in AML and their respective protocols, there is significant variation in the imaging modality and median follow-up duration. The median follow-up in these studies ranged from 4.5 to 96 months, with an average follow-up of 37 months [9]. CT is ideal for surveillance as it can clearly diagnose most AMLs as well as provides a better assessment of tumour size, fat content and risk of bleeding, but it carries the concern of radiation exposure. MRI, conversely, can be used for AMLs with less to no fat, it avoids radiation but is more costly and less readily available than CT. In small slowly growing AMLs US may also be used for surveillance provided a baseline US study is available for comparison. It is essential to balance the benign nature of AMLs with the risk of complications like bleeding, with the help of effective imaging and tailored surveillance. With improved diagnostic imaging and individualised follow-up strategies the goal is ensuring optimal care while minimising unnecessary interventions.
References
1. Nelson CP, Sanda MG. Contemporary diagnosis and management of renal AML. J Urol 2002;168(4 Pt 1):1315–25.
2. Lee KH, Tsai HY, Kao YT, et al. Clinical behavior and management of three types of renal AML’s. J Formos Med Assoc 2019;118(1 Pt 1):162–9.
3. Boorjian SA, Sheinin Y, Crispen PL, et al. Hormone receptor expression in renal AML: clinicopathologic correlation. Urology 2008;72(4):927–32.
4. Sim JS, Seo CS, Kim SH, et al. Differentiation of small hyperechoic renal cell carcinoma from AML: computer-aided tissue echo quantification. J Ultrasound Med 1999;18(4):261–4.
5. Song S, Park BK, Park JJ. New radiologic classification of renal AML’s. Eur J Radiol 2016;85(10):1835–40.
6. Park BK. Renal AML based on new classification: how to differentiate it from renal cell carcinoma. AJR Am J Roentgenol 2019;212(3):582–8.
7. Park BK. Renal AML: radiologic classification and imaging features according to the amount of fat. AJR Am J Roentgenol 2017;209(4):826–35.
8. Parihar AS, Mhlanga J, Ronstrom C, et al. Diagnostic accuracy of 99mTc-sestamibi SPECT/CT for characterization of solid renal masses. J Nucl Med 2023;64(1):90–5.
9. Sistani G, Bjazevic J, Kassam Z, et al. The value of 99mTc-sestamibi single-photon emission computed tomography-computed tomography in the evaluation and risk stratification of renal masses. Can Urol Assoc J 2021;15(6):197–201.
10. Fernández-Pello S, Hora M, Kuusk T, et al. Management of sporadic renal AML’s: a systematic review of available evidence to guide recommendations from the European Association of Urology Renal Cell Carcinoma Guidelines Panel. Eur Urol Oncol 2020;3(1):57–72.
11. Kuusk T, Biancari F, Lane B, et al. Treatment of renal AML: pooled analysis of individual patient data. BMC Urol 2015;15:123.
12. Chan KE, Chedgy E, Bent CL, Turner KJ. Surveillance imaging for sporadic renal AML less than 40 mm: lessons learnt and recommendations from the experience of a large district general hospital. Ann R Coll Surg Engl 2018;100:480–4.
13. Maclean DF, Sultana R, Radwan R, et al. Is the follow-up of small renal AML’s a necessary precaution? Clin Radiol 2014;69:822–6.
14. Ouzaid I, Autorino R, Fatica R, et al. Active surveillance for renal AML: outcomes and factors predictive of delayed intervention. BJU Int 2014;114(3):412–17.
15. Dorin R, Jackson M, Cusano A, et al. Active surveillance of renal masses: an analysis of growth kinetics and clinical outcomes stratified by radiological characteristics at diagnosis. Int Braz J Urol 2014;40(5):669–76.
Declaration of competing interests: None declared.