The barrier function of the glycosaminoglycan (GAG) layer of the urothelium was identified by Parsons in 1975, and intravesical therapies to treat chronic inflammatory conditions of the bladder were developed soon after. However, the active role of the urothelium in normal physiology and in the genesis of lower urinary tract dysfunction has become apparent only in recent years [1].
The barrier function and cellular signalling properties have without doubt now established the urothelium as a first responder to various physiological and non-physiological stimuli or stress, be it mechanical, chemical, microbiological, or hormonal. As a corollary, various inflammatory and chronic conditions of the bladder, including the painful bladder syndrome / interstitial cystitis (BPS/IC), have been associated with barrier dysfunction.
Although significant advances have been made in this area, much remains to be known; what we do know is that it is a lot more complex than we previously thought. This review seeks to provide an update on the current clinical and pre-clinical evidence for intravesical GAG replenishment therapies.
The biology of the GAG layer
Structure of the urothelial barrier: The bladder acts as a reservoir during the storage phase of the micturition cycle and is therefore subjected to exposure to various urinary constituents and shear stress from large volume changes. The urothelium comprises a number of distinct layers of which the surface cell layer is composed of umbrella cells. The luminal surface of the umbrella cell membrane is covered by a specialised asymmetrical structure called a glycocalyx. This consists of several uroplakins, a GAG layer, tight junctions and adherence cell junctions – this collectively forms a permeability barrier for substances in the urine such as solutes, potassium and bacteria from entering the urothelium. This barrier is not impermeable: it is balanced with regards to inflow and outflow of substances. The vascularity of the bladder wall is a key factor in the preservation of barrier function.
Composition and function of the GAG layer: Animal cells ubiquitously display a large array of glycoproteins, glycolipids, and proteoglycans on their surface. These mediate many fundamental cellular processes, including cell-matrix and cell-cell adhesion, motility, growth and signalling. The GAG layer is composed of polyanionic chains of variable length constructed from repeating disaccharide units, which contain a hexosamine residue and a uronic acid. They are bound to a core protein to form a proteoglycan. Proteoglycans play a number of important roles in the extracellular matrix and on cell surfaces.
“Loss of barrier function has been associated with molecular and histological changes, and gene expression consequent to passage of irritant substances from the urine...”
Urothelial GAGs have a high density of negative charge at the surface which leads to a physical phenomenon of a strong ordering of water molecules at the surface of the bladder into a gel via a process known as electrostatic entrapment. Most uncharged low molecular weight solutes and macromolecular solutes cannot get through this. Loss of this barrier function has been associated with molecular and histological changes and gene expression consequent to passage of irritant substances from the urine into the urothelial cellular layer; these include recruitment of inflammatory cytokines, overexpression of pro-inflammatory genes and neural upregulation. However, although barrier dysfunction appears to play an important role in BPS/IC, and this has been demonstrated in several trials, a multi-factorial aetiology is likely. We also know that barrier dysfunction may be a secondary effect in some conditions such as acute spinal cord injury, hypoperfusion, ageing and hormonal changes [2-9].
Distribution of urothelial GAGs: In mammalian tissue there are four main structural families of GAGs: chondroitin and dermatan sulphates, heparins and heparan sulphates, hyaluronate, and keratan sulphate. They are distributed in the matrix of connective tissue all over the body, and on the surface cells of organ systems.
Chondroitin sulphate (CS) is the most abundant GAG located on the urothelial luminal surface and was found to contribute to barrier function in studies on both human and animal models [4-6]. CS is principally associated with protein cores to form proteoglycans. There are four different types of chondroitin sulphate, depending on the position of the sulphate complex which form a family of molecules that include aggrecan and versican, which are major components of the extracellular matrix and have an important role in maintaining the structural integrity of tissues. In animal models of IC, treatment with chondroitin sulphate has been demonstrated to lead to a reduction in inflammatory cells (neutrophils and mast cells), in addition to reconstitution of the barrier function.
Hyaluronic acid (HA, also known as sodium hyaluronate or hyaluran) is distinct in that it is unsulphated and is not usually bound covalently to a core protein. HA consists of large polymers that can reach a molecular weight between 500,000 and 4,000,000 Daltons and a chain length of 10,000nm. Some animal and in vitro studies suggest higher molecular weights are associated with increased viscosity and better barrier function, whereas lower molecular weight HA may be associated with better tissue penetration and theoretically a reduction in inflammatory activity. HA also has a high capacity to bind water: 1g of HA binds up to 6L of water, which imparts a ‘spacer’ function. HA has been shown to positively modulate urothelial permeability, exhibit anti-inflammatory activity, and stimulate sulphated GAG synthesis, with decreased secretion of the pro-inflammatory cytokines interleukins IL6 and IL-8 [6]. Other anti-inflammatory mechanisms may be involved.
Immunofluorescence assays on human and porcine bladders have found that heparan sulphate (HS) and CS were found at similar concentrations in the deeper urothelial layers whereas low amounts of HS were detected in the mucinous layer on the luminal side of the urothelial cells. Dermatan sulphate was found only in the sub-urothelium and deeper layers of the bladder wall [5]. Heparin does not naturally occur on the bladder surface [2,4]. Some studies have found different GAG distributions but the above is the consensus view.
Pre-clinical studies: The efficacy of GAG replenishment therapy has been supported by several animal and human in vitro studies. Epithelial paracellular permeability can be studied by several methods, including use of dyes or small molecules with and without radiofluorescein isothiocyanate labelling, and the less invasive but extremely sensitive and reliable transepithelial electrical resistance (TEER) measurement technique [5]. A high TEER value represents a tight barrier which can be studied by using means of denuding and replenishing the GAG layer. Use of specific agents (e.g. protamine, trypsin, dilute HCL) either removes all GAGs or act selectively as CS digesting enzymes, thereby creating a barrier defect and with significant decrease in urothelial TEER readings. There is adequate evidence that exogenously produced GAGs bind to damaged urothelium to ‘repair’ artificially induced barrier defects. The administered CS in animal and human models has not been observed to penetrate the deeper layers of the urothelium but was seen to extensively bind damaged areas and restore the permeability barrier function [10,11].
Clinical evidence for GAG therapy: GAG replenishment therapy has been used for over 40 years based on pre-clinical data and that from small open-label clinical trials. Various agents have been used intravesically and orally to enhance the GAG layer or produce a local anaesthetic effect. Over the years, cumulative clinical experience has demonstrated considerable success rates with intravesical GAG replenishment without significant toxicity, in spite of a large placebo response and lack of response in a significant proportion of patients. However, this treatment remains limited by the lack of high-quality clinical trial evidence [2,4,6,12]. The EAU Guidelines 2020 allocate a ‘weak’ strength rating for recommending intravesical GAG replacement therapy based on level 2b evidence for chondroitin sulphate and level 3 for intravesical heparin [12]. For intravesical lidocaine combined with sodium bicarbonate and intravesical pentosan polysulphate the level of evidence (LE) is 1b. The American Urological Association (AUA) guideline (2014) supports heparin as an ‘option’ as a grade C recommendation (low level of certainty) [13], as does the International Consultation on Incontinence (ICI) [4].
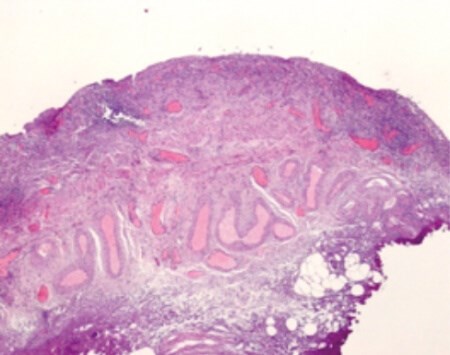
Figure 1: Urothelium demonstrating denudation of the GAG layer and infiltration with inflammatory cells.
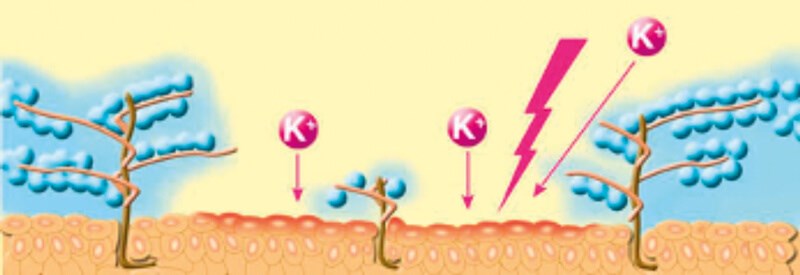
Figure 2: Schematic of the urothelium demonstrating electrostatic entrapment of water molecules by the GAG layer and the ability of potassium (K+) and other toxins to provoke an inflammatory response in its absence.
Treatment of BPS/IC
a) Chondroitin sulphate: CS is the most abundant GAG that is naturally present in the urothelium, and exogenously administered CS is taken up well by urothelium denuded of its GAG layer. In a recently published study, intravesical CS has been shown to improve the recovery of the damaged urothelial barrier in an in-vitro porcine model, confirming the mechanism of action of CS therapy [10]. Intravesical CS has been shown in animal models to reduce the recruitment of inflammatory cells (neutrophils and mast cells) in the suburothelial space without altering recruitment of CD45-positive lymphocytes, demonstrating an anti-inflammatory effect [6]. CS is extracted from animal tissue for pharmacological use, and is available in two different concentrations, 0.2% (40ml) and 2.0% (20ml) [6].
The clinical use of intravesical CS for BPS/IC is supported by a number of uncontrolled, open-label clinical trials which reported significant improvement in BPS/IC symptoms without any safety issues. A large study comprising a heterogeneous group of 286 patients with ‘chronic forms of cystitis’ (BPS/IC, OAB, radiation cystitis and recurrent bacterial cystitis), published in German, reported significant improvement in pain, urgency and frequency of micturition after three months’ treatment with 0.2% CS [15] with 82% providing a positive global rating after treatment. In a multicentre, community-based open-label study from Canada 48/53 patients who were naïve to intravesical 2.0% CS had a positive potassium sensitivity test. In all, 47% and 60% responded to intravesical 2.0% CS at week 10 and 24 of treatment respectively, with statistically significant reduction in mean symptom scores [16].
However, a multicentre, randomised, parallel group, double-blind study of 65 patients from the same team led by Curtis Nickel did not find a statistical difference between 2% CS instillation and intravesical vehicle control. Treatment was given for six weeks with a six-week follow-up; 39.4% of the active treatment group responded compared to 22.6% of the control group, as measured by the Global Response Assessment instrument and other secondary end-points. This study was however a pilot study and deliberately underpowered [17]. In a second double-blind, parallel group, randomised controlled trial (RCT) by the same authors 98 women were trialled for eight weekly treatments with 2.0% intravesical CS versus inactive control. Eighty-three percent of women completed the study, and from these 38% of the CS group and 31.3% of controls reported moderate or marked improvement [18]. Although more individuals in the active treatment group reported improvement in BPS/IC symptoms and pain scores, the results were not statistically significant. In a small open-label study of 18 patients treated with 0.2% CS for 13 months, 66.7% (n:12) showed a response with improvement in symptoms and 33% did not respond or withdrew from the study [19].
It is difficult to draw any firm conclusions from these very different results. It is obvious that there is a significant placebo effect with this treatment. The RCTs conducted with this agent were both small and purposely underpowered with the aim of establishing safety and efficacy information; the authors have estimated that a well-powered study would require 752 subjects per treatment group [18] – this underscores the inadequacy of the available data (the studies above recruited 53, 65 and 98 patients). In the open-label study [19], the average time to respond was two to twelve weeks and so perhaps the active treatment duration in the RCTs was a bit short.
In a meta-analysis using data from the two RCTs and one open-label study from Canada, published in 2013, the power of the available data was increased using an individual patient data (IPD) meta-analytical approach. From this analysis, the authors concluded that the chance of being a responder to treatment was 54% higher in the CS group [20]. CS has several advantages over other GAG products such as heparin, hyaluronate or pentosan polysulphate in being more biologically inert and less expensive [12]. Practitioners may recognise CS in the commercial names Gepana® (a non-viscous, pH neutral, 40ml solution of 0.2% CS in a pre-filled syringe) and Uracysta® (2% CS in 20mls). Intravesical CS is considered safe with no significant adverse effects: clinical trials reported mild adverse effects such as transient dysuria, rash, urethral pain, etc. equally in the control and active treatment arms.
b) Hyaluronic acid: HA, the only unsulphated GAG, is a muco-polysaccharide which is not bound by a core protein in the human bladder. Available commercially as Cystistata® (40mg sodium hyaluronate in a 50ml vial) and Hyacysta® (40mg/120mg in 50ml vials), HA has been in widespread use for many years with few adverse effects. Several reports have indicated its efficacy but there remains a paucity of good quality placebo-controlled RCT data [2,6,15]. An open label clinical trial of intravesical HA in 25 patients reported an initial response rate (complete + partial) of 56% at week four which increased to 71% at week 12 [21]. Another open-label trial reported longer term follow-up with a 50% complete symptomatic remission at five years, 41.7% had recurrent symptoms which improved on maintenance therapy [22]; these results are probably optimistic as they have not been replicated in other trials.
A review of nine studies on the outcomes of treatment of 172 IC patients with intravesical HA found a wide range of reported short-term response rates between 30% and 73%, with long-term responses approaching 55% [23]. The authors concluded that good quality data disease on HA which is specific to BPS/IC, blinded, placebo-controlled and adequately powered is unavailable, as did other reviews.
The beneficial effects of intravesical HA may be prolonged by combining intravesical HA with bladder distension [2,24]. In 2003 and 2004 two large placebo-controlled, double-blind phase 3 trials on intravesical HA with negative results were not published in the peer reviewed literature [6]. HA instillation employing electromotive drug administration (EMDA) improved outcomes at 6 and 12 months but not 24 months, reflecting a lack of long-term efficacy with this GAG therapy [6].
c) Heparin: Heparin is not normally found on the surface of the human bladder, but is purported to have properties similar to the GAG layer components. Clinical trials have reported efficacy in decreasing symptom scores, but no RCT evidence is available. No significant side-effects have been reported, and intravesical heparin does not affect systemic coagulation parameters. However, it may exacerbate local haemorrhage and so should not be used in the presence of haematuria [4]. In a study of 48 patients treated with 10,000 units of heparin in 10ml sterile water, 56% reported good clinical responses which in a proportion, was sustained over a nine-month period of maintenance therapy [25]. Intravesical heparin has been more frequently used in combination with other agents.
d) Pentosan polysulphate (PPS): This is a mucopolysaccharide similar to heparin, but its mechanism of action is not completely understood. It is theorised that it replaces the GAG layer. PPS is now primarily administered in an oral formulation but suffers from the disadvantage of a low concentration (1-3%) of the active drug reaching the bladder and a long lag time (three to six months) before clinical improvement is observed. It has therefore been used intravesically to circumvent these problems, but published trial data is sparse. However, there are two small placebo-controlled trials. In one study 4/10 patients responded with 300mg PPS in 50ml 0.9% sodium chloride administered twice weekly for three months versus 2/10 improvement in the placebo arm [26]. In another double-blind RCT 41 females were randomised to either intravesical and oral PPS (n:21) or a combination of oral PPS and intravesical placebo (n:20) for six weeks, with continuation of oral PPS for everyone for a further six weeks. There was a significant reduction in symptom scores in the treatment group vs. placebo at 12 weeks (-46% vs. -24%) with significant improvement at week 18 [27]. The ICI gives PPS a grade D recommendation based on level 4 evidence [4].
e) Combinations of GAG therapies: The combination of CS and HA forms a viscous agent, marketed under the brand name iAluril®, which may theoretically provide improved outcomes, with promising results shown in small patient cohorts. A phase III open-label randomised controlled study recruited 100 women with PBS/IC to receive a combination of 1.6% HA and 2.0% CS or 50% Dimethyl sulfoxide (DMSO) solution. Combination therapy was associated with a greater reduction in pain scores with fewer treatment-associated adverse effects [28]. DMSO is not a GAG and has consequently not been considered in the current review. Over the years, various anaesthetic ‘cocktails’ have been devised for intravesical use [29], the majority of which do not include GAG replenishment agents and have consequently not been considered here.
“This review exposes the dilemma of lack of high-quality clinical evidence to support intravesical GAG treatment whereas clinical experience suggests that this therapy replenishment has clearly observable benefits”
Conclusion
This review exposes the dilemma of lack of high-quality clinical evidence to support intravesical GAG treatment whereas clinical experience suggests that this therapy replenishment has clearly observable benefits; this is all the more relevant in the knowledge that there are no easy solutions for sufferers of BPS/IC. A meta-analysis of a limited number of 19 studies was conducted in an attempt to aid therapeutic decision-making from the heterogeneous data: response rates and effect size measurements were similar for high molecular weight (HMW) HA and 0.2% CS [30]. In the only head-to-head study between HA and 0.2% CS on 42 patients, both CS and HA yielded significant improvements in pain scores, IC symptom index and IC problem index scores, 24-hour frequency and nocturia at six months but CS was found to have statistically and clinically superior outcomes [31]. Whether there is truly such a difference between these agents remains to be confirmed by larger, well-powered randomised clinical trials.
The aetiology of BPS/IC is most likely multi-factorial, consequently GAG replenishment will not suit all patients. Future research needs to look at phenotyping individuals using an easily applicable tool and non-invasive diagnostic tests to identify those with deficiency of the GAG layer who would benefit from GAG therapy [6,17,18]. The heterogeneity of protocols used in various trials also suggest that the optimal installation schedule is not yet clearly defined.
TAKE HOME MESSAGE
-
The urothelium has active as well as protective barrier functions.
-
Chondroitin sulphate is the most abundant GAG in the urothelium.
-
Pre-clinical studies have conclusively demonstrated uptake of intravesically administered GAGs.
-
Good quality clinical trial data is lacking but long-term clinical experience suggest a considerable response to GAG replacement with low risk of adverse effects.
References
1. Birder LA, Ruggieri M, Takeda M, et al. How does the urothelium affect bladder function in health and disease? ICI-RS 2011. Neurourol Urodynam 2012;31:293-9.
2. Madersbacher H, van Ophoven A, van Kerrebroeck PEVA. GAG layer replenishment therapy for chronic forms of cystitis with intravesical glycosaminoglycans – a review. Neurourol Urodynam 2012;32:9-18.
3. Hurst RE, Rhodes S, Adamson PB, et al. Functional and structural characteristics of the glycosaminoglycans of the bladder luminal surface. J Urol 1987;138:433.
4. Hanno P (Chair). Bladder Pain Syndrome. In: 6th International Consultation on Incontinence. ICUD ICS; 2016:2243-6.
5. Janssen DAW, van Wijk XMR, Jansen KCFJ, et al. The distribution and function of chondroitin sulfate and other sulphated glycosaminoglycans in the human bladder and their contribution to the protective bladder barrier. J Urol 2013;189:336-42.
6. Wyndaele JJJ, Riedl C, Taneja R, et al. GAG replenishment therapy for bladder pain syndrome/interstitial cystitis. Neurourol Urodynam 2019;38:535-44.
7. Erickson DR, Sheykhnazari M, Ordille S, Bhavanandan VP. Increased urinary hyaluronic acid and interstitial cystitis. J Urol 1998;160:1282-4.
8. Hurst RE, Moldwin RM, Mulholland SG. Bladder defence molecules, urothelial differentiation, urinary biomarkers, and interstitial cystitis. Urology 2007;69(Suppl 4A):17-23.
9. Hauser PJ, Vangordan SB, et al. Abnormalities in expression of structural, barrier and differentiation related proteins, and chondroitin sulphate in feline and human interstitial cystitis. J Urol 2015;194:571-7.
10. Hauser PJ, Buethe DA, Califano J, et al. Restoring barrier function to acid damaged bladder by intravesical chondroitin sulfate. J Urol 2009;182:2477-82.
11. Kyker KD, Coffman J, Hurst RE. Exogenous glycosaminoglycans coat damaged bladder surfaces in experimentally damaged mouse bladder. BMC Urology 2005;5:4.
12. Engeler D, Baranowski AP, Berghmans B, et al. EAU Guidelines on Chronic Pelvic Pain. European Association of Urology; 2020.
https://uroweb.org/guideline/
chronic-pelvic-pain/
Accessed 28 March 2020.
13. Hanno PM, Burks DA, Quentin Clemens J, et al. American Urological Association (AUA) Guideline on Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. American Urological Association; 2014.
https://www.auanet.org/guidelines/
interstitial-cystitis-(ic/bps)-guideline.
Accessed 28 March 2020.
14. Rozenberg BB, Janssen DAW, Jansen CFJ, et al. Improving the barrier function of damaged cultured urothelium using chondroitin sulphate. Neurourol Urodynam 2020;39:558-64
15. Nordling J, van Ophoven A. Intravesical glycosaminoglycan replenishment with chondroitin sulphate in chronic forms of cystitis. A multi-national, multi-centre, prospective observational clinical trial. Arzneimittelforschung 2008;58:328–35.
16. Nickel JC, Egerdie B, Downey J, et al. A real-life multicentre clinical practice study to evaluate the efficacy and safety of intravesical chondroitin sulphate for the treatment of interstitial cystitis. BJU Int 2009;103:56.
17. Nickel JC, Egerdie B, Steinhoff G, et al. A multicentre, randomised, double-blind, parallel group pilot evaluation of the efficacy and safety of intravesical sodium chondroitin sulfate versus vehicle control in patients with interstitial cystitis/painful bladder syndrome. Urology 2010;76:804-9.
18. Nickel JC, Hanno P, Kumar K, et al. Second multicentre, randomized, double-blind, parallel-group evaluation of effectiveness and safety of intravesical sodium chondroitin sulfate compared with inactive vehicle control in subjects with interstitial cystitis/bladder pain syndrome. Urology 2012;79:1220-4.
19. Steinhoff G, Ittah B, Rowan S. The efficacy of chondroitin sulfate in treating interstitial cystitis. Can J Urol 2002;9:1454–8.
20. Thakkinstian A, Nickel JC. Efficacy of intravesical chondroitin sulphte in treatment of interstitial cystitis/bladder pain syndrome (IC/BPS): individual patient date (IPD) meta-analytical approach. Can Urol Assoc J 2013;7(5-6):195-200.
21. Morales A, Emerson L, nickel JC, et al. Intravescal hyaluronic acid in the treatment of refractory interstitial cystitis. Urology 1997;59(5A Suppl):111-13.
22. Engelhardt PF, Morakis N, Daha LK, et al. Long-term results of intravesical hyaluronan therapy in bladder pain syndrome/interstitial cystitis. Int Urogynecol J Pelvic Floor Dysfunct 2011;22:401-5.
23. Iavazzo C, Athanasiou S, Pitsouni E, Falagas ME. Hyaluronic Acid: An effective alternative treatment of Interstitial Cystitis, recurrent urinary tract infections, and hemorrhagic cystitis? Eur Urol 2007;51:1534-41.
24. Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: A systematic review. Eur Urol 2012;61:29-53.
25. Parsons CL, Housley T, Schmidt JD, Lebow D. Treatment of interstitial cystitis with intravesical heparin. Br J Urol 1994;73(5):504-7.
26. Bade JJ, Laseur M, Nieuwenburg A, et al. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol 1997;79:168–71.
27. Davis EL, El Khoudary SR, Talbott EO, et al. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol 2008;179:177–85.
28. Cervigni M, Sommariva M, Tenaglia R, et al. A randomized, open-label, multicentre study of the efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourol Urodynam 2017;36:1178-86.
29. Quillin RB, Erickson DR. Management of interstitial cystitis/bladder pain syndrome. A urology perspective. Urol Clin N Am 2012;39:389-96.
30. Barua JM, Arance I, Angulo JC, Riedl CR. A systematic review and meta-analysis on the efficacy of intravesical therapy for bladder pain syndrome/interstitial cystitis. Int Urogynecol J 2016;27:1137-47.
31. Gülpinar O, Esen B, Kayis A, et al. Clinical comparison of intravesical hyaluronic acid and chondroitin therapies in the treatment of bladder pain syndrome/interstitial cystitis. Neurourol Urodynam 2018;37:257-62.
Declaration of competing interests: None declared.