The menopause is a natural process of ageing when the ovaries completely stop producing reproductive hormones (oestrogen and other sex steroids), and there are no monthly periods for 12 consecutive months. It normally occurs between the ages of 45-55 years but can occur earlier due to certain surgical procedures (e.g., removal of ovaries), some cancer treatments (e.g., chemotherapy) or for a genetic reason.
It can be a challenging time for women with three quarters of women in the UK reporting that menopause has caused them to change their lives, struggle at work and in social situations and more than half say it has had a negative impact on their lives [1].
According to a recent systematic review the global prevalence of any lower urinary tract symptoms (LUTS) has been reported as 63.2% [2]. However, prevalence and severity of LUTS such as frequency, urgency, incontinence, and recurrent urinary tract infections increases around the menopause [3]. This has a further negative impact on quality of life for women. As the population is ageing and living longer, women are now spending an average of a third of their lifespan in a post-menopausal state.

This article aims to highlight the effect of ageing and loss of oestrogen on the urogenital tract and the problems that this may cause. It will address the pathophysiology of the vagina and lower urinary tract to help understand what changes happen to the tissues and structure at the time of the menopause. It will present the signs and symptoms to consider for women presenting to lower urinary tract services. Finally, it will discuss treatments in line with national guidelines, and additional sources of help and information will be shared.
Pathophysiology of the vagina
The vagina consists of three tissue layers: the muscularis, the lamina propria and the vaginal epithelium [4]. All three layers and the other tissues in the genitourinary tract (e.g., bladder and urethra) are rich in oestrogen receptors and responsive to sex steroids [5]. In the vagina, the pink colouration, moisture and rugae are dependent on the thickness of the muscularis and squamous vaginal epithelium and this is maintained by oestrogen [6]. Following the onset of menopause, the loss of vaginal rugal folds and the thinning of the epithelium is due to the breakdown of the collagen support of the vaginal epithelium [7]. Vaginal wall perfusion and muscle tone is affected by the vascular and non-vascular smooth muscle in the sub epithelial layers of the vagina – the growth and function of this smooth muscle is regulated by oestrogen [8]. The subepithelial vasculature is also a major contributor to vaginal moisture so lack of oestrogen here can lead to vaginal dryness [9].
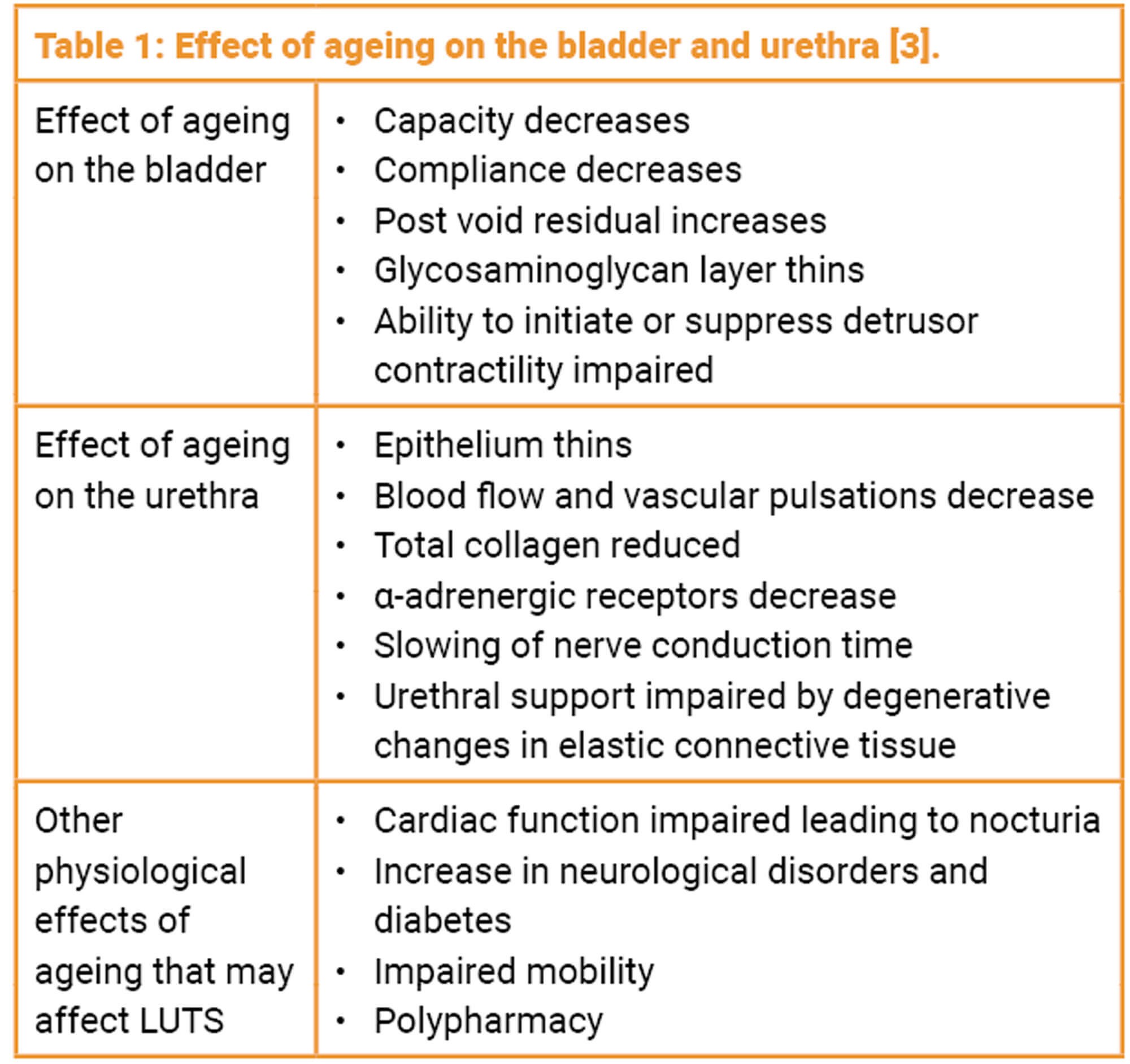
The urethra and bladder are derived from the same embryonic origin as the distal vagina and also have high levels of estrogen receptors and therefore post menopause will experience similar problems related to reduced blood flow, decrease in superficial and intermediate epithelial cells and loss of supporting structures which can lead to symptoms such as dysuria, increased bladder sensations and incontinence [10]. These symptoms can be compounded by the effect of ageing on the bladder and the urethra, as demonstrated in Table 1.
Genitourinary syndrome of menopause (GSM)
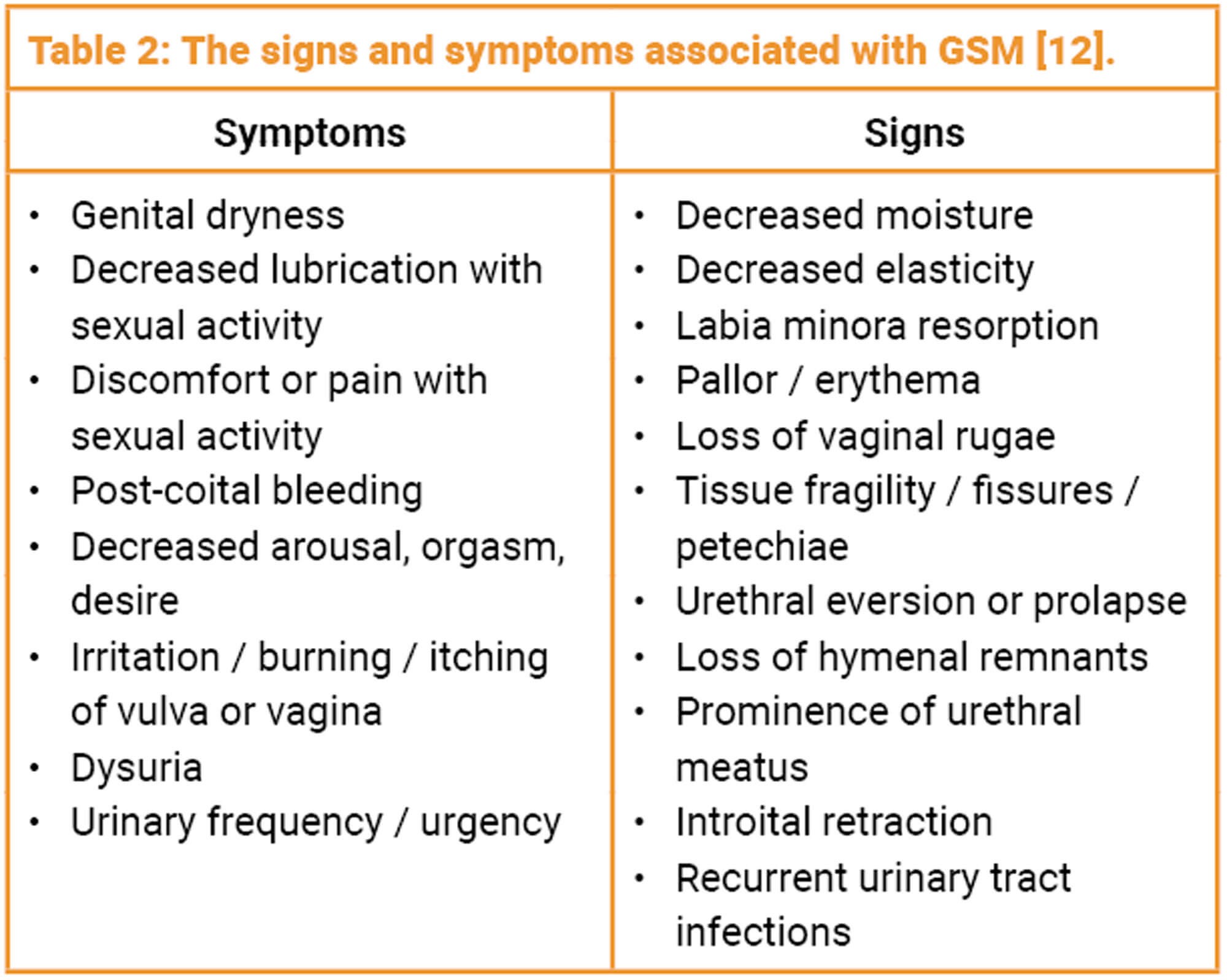
Atrophy of the vulva, vagina, lower urinary tract and supporting pelvic structures can be caused by prolonged oestrogen deprivation and results in a range of genitourinary symptoms including discomfort, pain, and impairment of sexual function and has been shown to negatively impact on quality of life [11]. Some women may start to develop symptoms in the perimenopause period but others may not be bothered until 10 years post menopause. In 2014 a consensus on vaginal atrophy terminology was published and GSM is the term that describes a collection of signs and symptoms associated with a decrease in oestrogen and other sex steroids involving changes to the labia majora / minora, clitoris, vestibule / introitus, vaginal, urethra and bladder (Table 2) [12].
The prevalence of GSM increases with age with one study suggesting it ranges from 64.7% to 84.2%, starting from one to six years after menopause [13]. Many women may only experience one mild symptom such as vaginal dryness whereas another may report severe symptoms affecting all the pelvic structures.
Assessment of GSM
Diagnosis of GSM is mainly through subjective assessment of symptoms and vaginal examination however, objective assessments (measurement of vaginal maturation index and vaginal pH) can be used but only tend to be performed in clinical research [14]. Observations during examination may include loss of pubic hair and definition to the labia, diminished vaginal rugae, smoother pale appearance to the tissue, mucosal defects including petichiae, microfissues, ulceration and inflammation. Vaginal narrowing, shortening and even obliteration of the vaginal vault can be the result of severe atrophy. For all postmenopausal women presenting to services with LUTS, an assessment of oestrogen status and treatment as appropriate is recommended as part of initial management [15].
For these women alongside the standard assessment of LUTS, past medical, obstetric and surgical history, medication use including prescribed, over the counter and herbal supplements, questions should be asked regarding other possible symptoms of GSM including vaginal dryness, itching, discharge and sexual symptoms. Women should also be asked about their age of menopause, if they have any other menopausal symptoms and if they are using or have previously used hormone replacement therapy (HRT).
Treatment of GSM
The primary goal of treatment of GSM is to reverse the atrophic changes and to improve or alleviate symptoms. Vaginal moisturisers and lubricants are available over the counter and can improve vaginal dryness and comfort but do not reverse changes. General health promotion e.g., vulval skin care, smoking cessation, maintaining a healthy weight, regular aerobic exercise and pelvic floor exercises are also advised.
Local vaginal oestrogen therapy lowers vaginal pH, thickens the epithelium, increases blood flow and improves vaginal lubrication [6]. A systematic review by Weber et al. confirmed the evidence for and positive effect of local oestrogen therapy in the treatment of LUTS [14].
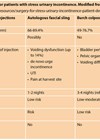
Vaginal oestrogens are the preferred treatment for GSM as lower doses can be used and there is limited systemic absorption, so the risks associated with oestrogen replacement are reduced (Table 3).

A review of all clinical studies assessing systemic absorption with vaginal oestriol confirmed that, although there was a small peak in the first two weeks of use, serum oestradiol levels remained within postmenopausal norms and after long-term use (three months) did not exceed baseline levels [16]. In 2015 the National Institute for Health & Care Excellence (NICE) published guidance for the diagnosis and management of menopause which recommended that clinicians should offer vaginal oestrogens to women with urogenital atrophy and continue treatment for as long as needed [17].
Local oestrogen therapy should be used with caution in women with a history of breast cancer and advice should be sought from the local oncology team. It should also be used with caution in women with arterial thromboembolic disease, venous thromboembolism, undiagnosed vaginal bleeding and thrombophilic disorders however, these are no longer considered definitive contraindications. For women who report other menopausal symptoms such as vasomotor symptoms, mood and memory changes, systemic oestrogens will be needed to treat these symptoms but 40% of women on systemic oestrogens still report GSM symptoms so may be on both systemic and vaginal oestrogens.
There are several challenges faced when prescribing hormone replacement therapy in the form of local oestrogens for the treatment of GSM. Over recent years the media have influenced women’s perceptions by perhaps exaggerating the risk of breast cancer, heart disease and stroke from use of topical oestrogen, without clarification of particular at-risk groups or quantification of specific risks. As such it has been reported that the fear of hormones was common in postmenopausal women using vaginal prescription products [18]. However, it has been confirmed that local treatment of vaginal atrophy is not associated with these possible risks of systemic HRT [6] and that adverse effects from vaginal oestrogen are very rare [17]. As part of the recent NHS England National Menopause Care Improvement Programme, not only have prescription costs associated with HRT therapy been reduced, but vaginal oestrogens are now available to buy over the counter without the need for a prescription.
For women in whom vaginal oestrogens are contraindicated or do not provide relief, there are other pharmacological treatments available for GSM symptoms e.g., ospemiphene and dehydroepiandrosterone (DHEA) but referral to a specialist menopause / urogynaecology service would be recommended prior to considering these options.
Conclusion
GSM is a prevalent condition that can significantly impact on bladder symptoms at the time of menopause and throughout the postmenopausal lifespan. For all women seeking help for LUTS, assessment of oestrogen status, vaginal examination for signs of GSM and appropriate treatment should be part of an initial management plan. It is also important to remember that GSM is a chronic condition and as such treatment is often required long term and symptoms often come back if treatment is stopped [17].
TAKE HOME MESSAGE
-
Genitourinary syndrome of menopause (GSM) is a common condition that can cause or worsen lower urinary tract symptoms (LUTS).
-
All post-menopausal women should be examined and assessed for GSM when presenting with LUTS.
-
Treatment of GSM can improve or cure LUTS.
References
1. British Menopause Society. A woman’s relationship with the menopause is complicated. 2020
https://www.womens-health-concern.org/
wp-content/uploads/2020/09/BMS-Infographic
-A-womans-relationship-with-the
-menopause-SEPT2020-B.pdf
[accessed 6 June 2023].
2. Huang J, Chan CK, Yee S, et al. Global burden and temporal trends of lower urinary tract symptoms: a systematic review and meta-analysis. Prostate Cancer & Prostatic Diseases 2022; [Epub ahead of print].
3. Hillard T. The postmenopausal bladder. Menopause International 2010;16(2):74-80.
4. Tzur T, Yohai D, Weintraub AY. The role of local estrogen therapy in the management of pelvic floor disorders. Climacteric 2016;19(2):162-71.
5. Kelley C. Estrogen and its effect on vaginal atrophy in post-menopausal women. Urological Nursing 2007;27(1):40-5.
6. Sturdee DW, Panay N. Recommendations for the management of postmenopausal vaginal atrophy. Climacteric 2010;13(6):509-22.
7. Moalli PA, Talarico LC, Sung VW, et al. Impact of menopause on collagen subtypes in the arcus tendineous fasciae pelvis. American Journal of Obstetric Gynecology 2004;190:620-7.
8. Kim SW, Kim NN, Jeong SJ, et al. Modulation of rat vaginal blood flow and estrogen receptor by estradiol. Journal of Urology 2004;172(4):1538-43.
9. Goldstein I, Dicks B, Kim NN, Hartzell R. Multidisciplinary overview of vaginal atrophy and associated genitourinary symptoms in postmenopausal women. Sexual Medicine 2013;1(2):44-53.
10. Archer DF. Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause 2010;17:194-203.
11. Sousa MS, Peate M, Jarvis S, et al. A clinical guide to the management of genitourinary symptoms in breast cancer survivors on endocrine therapy. Therapeutic Advances in Medical Oncology 2017;9(4):269-85.
12. Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and The North American Menopause Society. The Journal of Sexual Medicine 2014;11(12):2865-72.
13. Palma F, Volpe A, Villa P, Cagnacci A. Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study. Maturitas 2016;83:40-4.
14. Weber MA, Kleijn MH, Langendam M, et al. Local oestrogen for pelvic floor disorders: a systematic review. PLoS One 2015;10(9):e0136265.
15. Cardozo L, Rovner E, Wagg A, et al. (Eds). Incontinence 7th Edition Bristol, UK: International Continence Society; 2023.
16. Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10μg 17β-estradiol vaginal tablets. Climacteric 2010;13:219-27.
17. National Institute for Health and Care Excellence (NICE). NG23, Menopause: diagnosis and management. London, UK; NICE: 2015.
18. Nappi RE, Palacios S, Panay N, et al. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey. Climacteric 2016;19(2):188-97.
Additional sources of information
There are several websites that provide reputable information for women and health care providers, listed below. The patient facing website for the International Urogynecology Association (IUGA) –
https://www.yourpelvicfloor.org/ – has patient information leaflets on vaginal oestrogen therapy that can be downloaded in multiple languages for use in clinics.
https://www.nhs.uk/conditions/menopause
https://thebms.org.uk/publications/tools-for-clinicians
https://www.yourpelvicfloor.org/media/
Low-Dose_Vaginal_Estrogen_Therapy.pdf
https://www.skinhealthinfo.org.uk/condition/care-of-vulval-skin
Declaration of competing interests: None declared.